Early Life to Adult Brain Lipidome Dynamic: A Temporospatial Study Investigating Dietary Polar Lipid Supplementation Efficacy
- PMID: 35967787
- PMCID: PMC9364220
- DOI: 10.3389/fnut.2022.898655
Early Life to Adult Brain Lipidome Dynamic: A Temporospatial Study Investigating Dietary Polar Lipid Supplementation Efficacy
Abstract
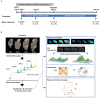
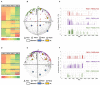
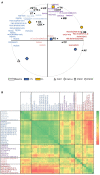
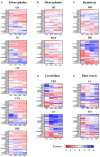
The lipid composition of the brain is well regulated during development, and the specific temporospatial distribution of various lipid species is essential for the development of optimal neural functions. Dietary lipids are the main source of brain lipids and thus contribute to the brain lipidome. Human milk is the only source of a dietary lipids for exclusively breastfed infant. Notably, it contains milk fat globule membrane (MFGM) enriched in polar lipids (PL). While early life is a key for early brain development, the interplay between dietary intake of polar lipids and spatial dynamics of lipid distribution during brain development is poorly understood. Here, we carried out an exploratory study to assess the early postnatal temporal profiling of brain lipidome between postnatal day (PND) 7 and PND 50 using matrix-assisted laser desorption ionization as a mass spectrometry imaging (MALDI-MSI) in an in vivo preclinical model. We also assessed the effect of chronic supplementation with PL extracted from alpha-lactalbumin-enriched whey protein concentrate (WPC) containing 10% lipids, including major lipid classes found in the brain (37% phospholipids and 15% sphingomyelin). MALDI-MSI of the spatial and temporal accretion of lipid species during brain development showed that the brain lipidome is changing heterogeneously along time during brain development. In addition, increases in 400+ PL supplement-dependent lipids were observed. PL supplementation had significant spatial and temporal effect on specific fatty esters, glycerophosphocholines, glycerophosphoethanolamines, and phosphosphingolipids. Interestingly, the average levels of these lipids per brain area tended to be constant in various brain structures across the age groups, paralleling the general brain growth. In contrast, other lipids, such as cytidine diphosphate diacylglycerol, diacylglycerophosphates, phosphocholines, specific ether-phosphoethanolamines, phosphosphingolipids, glycerophosphoinositols, and glycerophosphoserines showed clear age-dependent changes uncoupled from the general brain growth. These results suggest that the dietary PL supplementation may preferentially provide the building blocks for the general brain growth during development. Our findings add to the understanding of brain-nutrient relations, their temporospatial dynamics, and potential impact on neurodevelopment.
Keywords: MALDI-MSI; brain development; phospholipids; polar lipids; sphingolipids.
Copyright © 2022 Oliveira, Koshibu, Rytz, Giuffrida, Sultan, Patin, Gaudin, Tomezyk, Steiner and Schneider.
Conflict of interest statement
MO, KK, AR, FG, AP, SS, PS, and NS were employed by the company Société des Produits Nestlé SA. MG and AT were employed by ImaBiotech SAS.
Figures





Similar articles
-
Effects of Post-natal Dietary Milk Fat Globule Membrane Polar Lipid Supplementation on Motor Skills, Anxiety, and Long-Term Memory in Adulthood.Front Nutr. 2021 Nov 16;8:737731. doi: 10.3389/fnut.2021.737731. eCollection 2021. Front Nutr. 2021. PMID: 34869518 Free PMC article.
-
Supplementation of milk polar lipids to obese dams improves neurodevelopment and cognitive function in male offspring.FASEB J. 2021 Apr;35(4):e21454. doi: 10.1096/fj.202001974RRR. FASEB J. 2021. PMID: 33749945
-
Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood.Br J Nutr. 2016 Jun;115(11):1930-7. doi: 10.1017/S0007114516001082. Epub 2016 Apr 4. Br J Nutr. 2016. PMID: 27040581 Free PMC article.
-
Dietary Polar Lipids and Cognitive Development: A Narrative Review.Adv Nutr. 2019 Nov 1;10(6):1163-1176. doi: 10.1093/advances/nmz051. Adv Nutr. 2019. PMID: 31147721 Free PMC article. Review.
-
Surface analysis of lipids by mass spectrometry: more than just imaging.Prog Lipid Res. 2013 Oct;52(4):329-53. doi: 10.1016/j.plipres.2013.04.005. Epub 2013 Apr 24. Prog Lipid Res. 2013. PMID: 23623802 Review.
Cited by
-
Milk Fat Globule Membranes for Mental Health across the Human Lifespan.Foods. 2024 May 24;13(11):1631. doi: 10.3390/foods13111631. Foods. 2024. PMID: 38890860 Free PMC article. Review.
-
Spatially heterogeneous lipid dysregulation in tuberculous meningitis.Neurobiol Dis. 2024 Nov;202:106721. doi: 10.1016/j.nbd.2024.106721. Epub 2024 Nov 1. Neurobiol Dis. 2024. PMID: 39489454 Free PMC article.
-
Dynamic Interplay between Social Brain Development and Nutrient Intake in Young Children.Nutrients. 2023 Aug 28;15(17):3754. doi: 10.3390/nu15173754. Nutrients. 2023. PMID: 37686785 Free PMC article.
References
LinkOut - more resources
Full Text Sources

