Pathological and protective roles of dendritic cells in Mycobacterium tuberculosis infection: Interaction between host immune responses and pathogen evasion
- PMID: 35967869
- PMCID: PMC9366614
- DOI: 10.3389/fcimb.2022.891878
Pathological and protective roles of dendritic cells in Mycobacterium tuberculosis infection: Interaction between host immune responses and pathogen evasion
Abstract
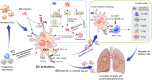
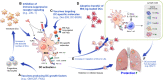
Dendritic cells (DCs) are principal defense components that play multifactorial roles in translating innate immune responses to adaptive immunity in Mycobacterium tuberculosis (Mtb) infections. The heterogeneous nature of DC subsets follows their altered functions by interacting with other immune cells, Mtb, and its products, enhancing host defense mechanisms or facilitating pathogen evasion. Thus, a better understanding of the immune responses initiated, promoted, and amplified or inhibited by DCs in Mtb infection is an essential step in developing anti-tuberculosis (TB) control measures, such as host-directed adjunctive therapy and anti-TB vaccines. This review summarizes the recent advances in salient DC subsets, including their phenotypic classification, cytokine profiles, functional alterations according to disease stages and environments, and consequent TB outcomes. A comprehensive overview of the role of DCs from various perspectives enables a deeper understanding of TB pathogenesis and could be useful in developing DC-based vaccines and immunotherapies.
Keywords: Mycobacterium tuberculosis; dendritic cells; host-directed strategy; pathogenesis; protective immunity; vaccine.
Copyright © 2022 Kim and Shin.
Conflict of interest statement
The authors declare that this review was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Natural and trained innate immunity against Mycobacterium tuberculosis.Immunobiology. 2020 May;225(3):151951. doi: 10.1016/j.imbio.2020.151951. Epub 2020 Apr 27. Immunobiology. 2020. PMID: 32423788 Review.
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
Innate immunity in tuberculosis: host defense vs pathogen evasion.Cell Mol Immunol. 2017 Dec;14(12):963-975. doi: 10.1038/cmi.2017.88. Epub 2017 Sep 11. Cell Mol Immunol. 2017. PMID: 28890547 Free PMC article. Review.
-
New insights into the evasion of host innate immunity by Mycobacterium tuberculosis.Cell Mol Immunol. 2020 Sep;17(9):901-913. doi: 10.1038/s41423-020-0502-z. Epub 2020 Jul 29. Cell Mol Immunol. 2020. PMID: 32728204 Free PMC article. Review.
-
Genetic-and-Epigenetic Interspecies Networks for Cross-Talk Mechanisms in Human Macrophages and Dendritic Cells during MTB Infection.Front Cell Infect Microbiol. 2016 Oct 18;6:124. doi: 10.3389/fcimb.2016.00124. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27803888 Free PMC article.
Cited by
-
Microbiota and Immunity during Respiratory Infections: Lung and Gut Affair.Int J Mol Sci. 2024 Apr 5;25(7):4051. doi: 10.3390/ijms25074051. Int J Mol Sci. 2024. PMID: 38612860 Free PMC article. Review.
-
Vaccine development against tuberculosis before and after Covid-19.Front Immunol. 2023 Nov 15;14:1273938. doi: 10.3389/fimmu.2023.1273938. eCollection 2023. Front Immunol. 2023. PMID: 38035095 Free PMC article. Review.
-
The Role of Inflammation in the Pathogenesis of Comorbidity of Chronic Obstructive Pulmonary Disease and Pulmonary Tuberculosis.Int J Mol Sci. 2025 Mar 7;26(6):2378. doi: 10.3390/ijms26062378. Int J Mol Sci. 2025. PMID: 40141021 Free PMC article. Review.
-
Characterizing the immune response to Mycobacterium tuberculosis: a comprehensive narrative review and implications in disease relapse.Front Immunol. 2024 Nov 22;15:1437901. doi: 10.3389/fimmu.2024.1437901. eCollection 2024. Front Immunol. 2024. PMID: 39650648 Free PMC article. Review.
-
Immune-endocrine network in diabetes-tuberculosis nexus: does latent tuberculosis infection confer protection against meta-inflammation and insulin resistance?Front Endocrinol (Lausanne). 2024 Jan 24;15:1303338. doi: 10.3389/fendo.2024.1303338. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38327565 Free PMC article. Review.
References
-
- Alemãn M., de la Barrera S., Schierloh P., Yokobori N., Baldini M., Musella R., et al. . (2007). Spontaneous or mycobacterium tuberculosis-induced apoptotic neutrophils exert opposite effects on the dendritic cell-mediated immune response. Eur. J. Immunol. 37, 1524–1537. doi: 10.1002/eji.200636771 - DOI - PubMed
-
- Amaral J. J., Antunes L. C., De Macedo C. S., Mattos K. A., Han J., Pan J., et al. . (2013). Metabonomics reveals drastic changes in anti-inflammatory/pro-resolving polyunsaturated fatty acids-derived lipid mediators in leprosy disease. PloS Negl. Trop. Dis. 7, e2381. doi: 10.1371/journal.pntd.0002381 - DOI - PMC - PubMed
-
- Amir M., Aqdas M., Nadeem S., Siddiqui K. F., Khan N., Sheikh J. A., et al. . (2017). Diametric role of the latency-associated protein Acr1 of Mycobacterium tuberculosis in modulating the functionality of pre- and post-maturational stages of dendritic cells. Front. Immunol. 8, 624. doi: 10.3389/fimmu.2017.00624 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

