Receptor for advanced glycation end-products and child neglect in mice: A possible link to postpartum depression
- PMID: 35967921
- PMCID: PMC9363643
- DOI: 10.1016/j.cpnec.2022.100146
Receptor for advanced glycation end-products and child neglect in mice: A possible link to postpartum depression
Abstract
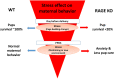
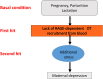
The receptor for advanced glycation end-products (RAGE), a pattern recognition molecule, has a role in the remodeling of vascular endothelial cells mainly in lungs, kidney and brain under pathological conditions. We recently discovered that RAGE binds oxytocin (OT) and transports it to the brain from circulation on neurovascular endothelial cells. We produced knockout mice of the mouse homologue of the human RAGE gene, Ager, designated RAGE KO mice. In RAGE KO mice, while hyperactivity has been reported in male mice, maternal behavior was impaired in female mice. After an additional stress, deficits in pup care were observed in RAGE KO mother mice. This resulted in pup death within 1-2 days, suggesting that RAGE plays a critical role during the postpartum period. Thus, RAGE seems to be important in the manifestation of normal maternal behavior in dams. In this review, we summarize the significance of brain OT transport by RAGE and propose that RAGE-dependent OT can dampen stress signals during pregnancy, delivery and early postpartum periods. To the best of our knowledge, there have been no previous articles on these RAGE-dependent results. Based on these results in mice, we discuss a potential critical role of RAGE in emotion swings at the puerperium (peripartum) and postpartum periods in women.
Keywords: Maternal behavior; Oxytocin; Peripartum; Postpartum; Puerperium; RAGE; Stress.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Receptor for advanced glycation end-products (RAGE) plays a critical role in retrieval behavior of mother mice at early postpartum.Physiol Behav. 2021 Jun 1;235:113395. doi: 10.1016/j.physbeh.2021.113395. Epub 2021 Mar 21. Physiol Behav. 2021. PMID: 33757778
-
Oxytocin transported from the blood across the blood-brain barrier by receptor for advanced glycation end-products (RAGE) affects brain function related to social behavior.Peptides. 2024 Aug;178:171230. doi: 10.1016/j.peptides.2024.171230. Epub 2024 Apr 26. Peptides. 2024. PMID: 38677620 Review.
-
Transport of oxytocin to the brain after peripheral administration by membrane-bound or soluble forms of receptors for advanced glycation end-products.J Neuroendocrinol. 2021 Mar;33(3):e12963. doi: 10.1111/jne.12963. J Neuroendocrinol. 2021. PMID: 33733541
-
An improved sample extraction method reveals that plasma receptor for advanced glycation end-products (RAGE) modulates circulating free oxytocin in mice.Peptides. 2021 Dec;146:170649. doi: 10.1016/j.peptides.2021.170649. Epub 2021 Sep 17. Peptides. 2021. PMID: 34543678
-
Oxytocin Dynamics in the Body and Brain Regulated by the Receptor for Advanced Glycation End-Products, CD38, CD157, and Nicotinamide Riboside.Front Neurosci. 2022 Jul 7;16:858070. doi: 10.3389/fnins.2022.858070. eCollection 2022. Front Neurosci. 2022. PMID: 35873827 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

