The effects of antimicrobials and lipopolysaccharide on acute immune responsivity in pubertal male and female CD1 mice
- PMID: 35967925
- PMCID: PMC9363646
- DOI: 10.1016/j.cpnec.2022.100147
The effects of antimicrobials and lipopolysaccharide on acute immune responsivity in pubertal male and female CD1 mice
Abstract
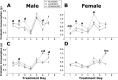
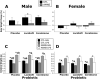
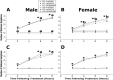
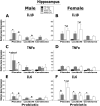
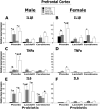
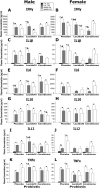
Exposure to stress during critical periods of development-such as puberty-is associated with long-term disruptions in brain function and neuro-immune responsivity. However, the mechanisms underlying the effect of stress on the pubertal neuro-immune response has yet to be elucidated. Therefore, the objective of the current study was to investigate the effect antimicrobial and lipopolysaccharide (LPS) treatments on acute immune responsivity in pubertal male and female mice. Moreover, the potential for probiotic supplementation to mitigate these effects was also examined. 240 male and female CD1 mice were treated with one week of antimicrobial treatment (mixed antimicrobials or water) and probiotic treatment (L. rhamnosis R0011 and L. helveticus R0052 or L. helveticus R0052 and B. longum R0175) or placebo at five weeks of age. At six weeks of age (pubertal stress-sensitive period), the mice received a single injection of LPS or saline. Sickness behaviours were assessed, and mice were euthanized 8 h post-injection. Brain, blood, and intestinal samples were collected. The results indicated that the antimicrobial treatment reduced sickness behaviours, and potentiated LPS-induced plasma cytokine concentrations and pro-inflammatory markers in the pre-frontal cortex (PFC) and hippocampus, in a sex-dependent manner. However, probiotics reduced LPS-induced plasma cytokine concentrations along with hippocampal and PFC pro-inflammatory markers in a sex-dependent manner. L. rhamnosis R0011 and L. helveticus R0052 treatment also mitigated antimicrobial-induced plasma cytokine concentrations and sickness behaviours. These findings suggest that the microbiome is an important modulator of the pro-inflammatory immune response during puberty.
Keywords: Inflammation; LPS; Microbiome; Probiotics; Puberty; Sex differences.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
None.
Figures
References
-
- Agostini A., Yuchun D., Li B., Kendall D.A., Pardon M.-C. Sex-specific hippocampal metabolic signatures at the onset of systemic inflammation with lipopolysaccharide in the APPswe/PS1dE9 mouse model of Alzheimer's disease. Brain Behav. Immun. 2020;83:87–111. doi: 10.1016/j.bbi.2019.09.019. - DOI - PMC - PubMed
-
- Ait-Belgnaoui A., Payard I., Rolland C., Harkat C., Braniste V., Théodorou V., Tompkins T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motility. 2018;24(1):138–146. doi: 10.5056/jnm16167. - DOI - PMC - PubMed
-
- Brown A.P., Dinger N., Levine B.S. Stress produced by gavage administration in the rat. Contemp. Top. Lab. Anim. Sci. 2000;39(1):17–21. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

