Establishment of blood glycosidase activities and their excursions in sepsis
- PMID: 35967980
- PMCID: PMC9364217
- DOI: 10.1093/pnasnexus/pgac113
Establishment of blood glycosidase activities and their excursions in sepsis
Erratum in
-
Correction to: Establishment of blood glycosidase activities and their excursions in sepsis.PNAS Nexus. 2024 Jan 30;3(1):pgad463. doi: 10.1093/pnasnexus/pgad463. eCollection 2024 Jan. PNAS Nexus. 2024. PMID: 38292545 Free PMC article.
-
Correction to: Establishment of blood glycosidase activities and their excursions in sepsis.PNAS Nexus. 2025 Aug 26;4(8):pgaf265. doi: 10.1093/pnasnexus/pgaf265. eCollection 2025 Aug. PNAS Nexus. 2025. PMID: 40874026 Free PMC article.
Abstract
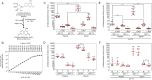
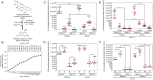
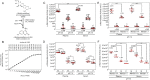
Glycosidases are hydrolytic enzymes studied principally in the context of intracellular catabolism within the lysosome. Therefore, glycosidase activities are classically measured in experimentally acidified assay conditions reflecting their low pH optima. However, glycosidases are also present in the bloodstream where they may retain sufficient activity to participate in the regulation of glycoprotein half-lives, proteostasis, and disease pathogenesis. We have, herein, established at physiological pH 7.4 in blood plasma and sera the normal ranges of four major glycosidase activities essential for blood glycoprotein remodeling in healthy mice and humans. These activities included β-galactosidase, β-N-acetylglucosaminidase, α-mannosidase, and α-fucosidase. We have identified their origins to include the mammalian genes Glb1, HexB, Man2a1, and Fuca1. In experimental sepsis, excursions of glycosidase activities occurred with differences in host responses to discrete bacterial pathogens. Among similar excursions in human sepsis, the elevation of β-galactosidase activity was a prognostic indicator of increased likelihood of patient death.
© The Author(s) 2022. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Bacterial vampirism mediated through taxis to serum.Elife. 2024 May 31;12:RP93178. doi: 10.7554/eLife.93178. Elife. 2024. PMID: 38820052 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Genomic surveillance for multidrug-resistant or hypervirulent Klebsiella pneumoniae among United States bloodstream isolates.BMC Infect Dis. 2022 Jul 7;22(1):603. doi: 10.1186/s12879-022-07558-1. BMC Infect Dis. 2022. PMID: 35799130 Free PMC article.
Cited by
-
Induced volatolomics to uncover new enzymatic hallmarks of precancerous lesions: a proof of concept on gastric preneoplasia in mice.Biochem Biophys Rep. 2025 May 30;43:102062. doi: 10.1016/j.bbrep.2025.102062. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40519703 Free PMC article.
-
Altered Glycosylation of PSA in Prostate Cancer Tissue.Prostate. 2025 Oct;85(14):1290-1298. doi: 10.1002/pros.70014. Epub 2025 Jul 9. Prostate. 2025. PMID: 40635354 Free PMC article.
-
Free oligosaccharides in serum.BBA Adv. 2025 Jan 10;7:100139. doi: 10.1016/j.bbadva.2025.100139. eCollection 2025. BBA Adv. 2025. PMID: 39897077 Free PMC article.
References
-
- Henrissat B, Davies G. 1997. Structural and sequence-based classification of glycoside hydrolyases. Curr Opin Struct Biol. 7:637–644. - PubMed
-
- Winchester B. 2005. Lysosomal metabolism of glycoproteins. Glycobiology. 15:1R–15R. - PubMed
-
- Durand P et al. 1977. Sialidosis (mucolipidosis I). Helv Paediatr Acta. 32:391–400. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

