Safety and efficacy of cannabidiol-cannabidiolic acid rich hemp extract in the treatment of refractory epileptic seizures in dogs
- PMID: 35967998
- PMCID: PMC9372618
- DOI: 10.3389/fvets.2022.939966
Safety and efficacy of cannabidiol-cannabidiolic acid rich hemp extract in the treatment of refractory epileptic seizures in dogs
Abstract
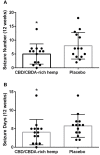
The use of cannabidiol (CBD) in childhood refractory seizures has become a common therapeutic approach for specific seizure disorders in human medicine. Similarly, there is an interest in using CBD, cannabidiolic acid (CBDA) or cannabinoid-rich hemp products in the treatment of idiopathic epilepsy in dogs. We aimed to examine a small cohort in a pilot investigation using a CBD and CBDA-rich hemp product for the treatment of refractory epileptic seizures in dogs. Fourteen dogs were examined in a 24-week randomized cross-over study being provided placebo or CBD/CBDA-rich hemp extract treatment at 2 mg/kg orally every 12 h for each 12-week arm of the study. Serum chemistry, complete blood counts, serum anti-seizure medication (ASM) concentrations and epileptic seizure frequency were followed over both arms of the cross-over trial. Results demonstrated that besides a mild increase in alkaline phosphatase, there were no alterations observed on routine bloodwork at 2, 6, and 12 weeks during either arm of the study. Epileptic seizure frequency decreased across the population from a mean of 8.0 ± 4.8 during placebo treatment to 5.0 ± 3.6 with CBD/CBDA-rich hemp extract (P = 0.02). In addition, epileptic seizure event days over the 12 weeks of CBD/CBDA-rich hemp treatment were 4.1 ± 3.4, which was significantly different than during the 12 weeks of placebo treatment (5.8 ± 3.1; P =0.02). The number of dogs with a 50% reduction in epileptic activity while on treatment were 6/14, whereas 0/14 had reductions of 50% or greater while on the placebo (P = 0.02). No differences were observed in serum zonisamide, phenobarbital or bromide concentrations while on the treatment across groups. Adverse events were minimal, but included somnolence (3/14) and transient increases in ataxia (4/14) during CBD/CBDA-rich hemp extract treatment; this was not significantly different from placebo. This further indicates that providing CBD/CBDA-rich hemp extract during refractory epilepsy (only partially responsive to ASM), in conjunction with other ASM appears safe. Based on this information, the use of 2 mg/kg every 12 h of a CBD/CBDA-rich hemp extract can have benefits in reducing the incidence of epileptic seizures, when used concurrently with other ASMs.
Keywords: cannabidiol; cannabidiolic acid; dog; phenobarbital; seizure; zonisamide.
Copyright © 2022 Garcia, Kube, Carrera-Justiz, Tittle and Wakshlag.
Conflict of interest statement
Author GG received a grant from Ellevet Sciences to complete the clinical trial outlines in the manuscript and is an Advisory Board Member at Ellevet Sciences. Authors JW and DT are consultants at Ellevet Sciences and also serve as advisory board members at Ellevet Sciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Tolerability of 2 and 4 mg/kg Dosing Every 12 Hour of a Cannabidiol- and Cannabidiolic Acid-Rich Hemp Extract on Mixed-Breed Dogs Utilized for Teaching in a Closed Colony.Animals (Basel). 2024 Jun 24;14(13):1863. doi: 10.3390/ani14131863. Animals (Basel). 2024. PMID: 38997975 Free PMC article.
-
Safety, Efficacy and Doxorubicin Pharmacokinetics During Cannabidiol/Cannabidiolic Acid Rich Hemp Oil Use in Dogs With Lymphoma Undergoing CHOP Chemotherapy.J Vet Intern Med. 2025 Jul-Aug;39(4):e70179. doi: 10.1111/jvim.70179. J Vet Intern Med. 2025. PMID: 40622742 Free PMC article.
-
Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract.Front Vet Sci. 2020 Sep 4;7:505. doi: 10.3389/fvets.2020.00505. eCollection 2020. Front Vet Sci. 2020. PMID: 33102539 Free PMC article.
-
Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis.Drugs. 2018 Nov;78(17):1791-1804. doi: 10.1007/s40265-018-0992-5. Drugs. 2018. PMID: 30390221
-
Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox-Gastaut Syndrome.CNS Drugs. 2021 Mar;35(3):265-281. doi: 10.1007/s40263-021-00807-y. Epub 2021 Mar 22. CNS Drugs. 2021. PMID: 33754312 Free PMC article.
Cited by
-
Cannabis sativa in veterinary medicine: Foundations and therapeutic applications.Can Vet J. 2024 Sep;65(9):948-958. Can Vet J. 2024. PMID: 39219599 Free PMC article. Review.
-
Danish dog owners' use and the perceived effect of unlicensed cannabis products in dogs.PLoS One. 2024 Jan 31;19(1):e0296698. doi: 10.1371/journal.pone.0296698. eCollection 2024. PLoS One. 2024. PMID: 38295012 Free PMC article.
-
Endocannabinoid system and phytocannabinoids in the main species of veterinary interest: a comparative review.Vet Res Commun. 2024 Oct;48(5):2915-2941. doi: 10.1007/s11259-024-10509-7. Epub 2024 Aug 20. Vet Res Commun. 2024. PMID: 39162768 Free PMC article. Review.
-
Pharmacokinetics, efficacy, and safety of cannabidiol in dogs: an update of current knowledge.Front Vet Sci. 2023 Jun 30;10:1204526. doi: 10.3389/fvets.2023.1204526. eCollection 2023. Front Vet Sci. 2023. PMID: 37456953 Free PMC article. Review.
-
Safety study of cannabidiol products in healthy dogs.Front Vet Sci. 2024 Mar 1;11:1349590. doi: 10.3389/fvets.2024.1349590. eCollection 2024. Front Vet Sci. 2024. PMID: 38496308 Free PMC article.
References
LinkOut - more resources
Full Text Sources

