Early Real-World Physician Experience with an Intracanalicular Dexamethasone Insert
- PMID: 35968052
- PMCID: PMC9365058
- DOI: 10.2147/OPTH.S372440
Early Real-World Physician Experience with an Intracanalicular Dexamethasone Insert
Abstract
Purpose: To describe the early real-world experience of physicians with an intracanalicular dexamethasone insert (DEX) in patients undergoing cataract surgery and to capture the clinical impact of adopting this therapy.
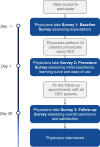
Patients and methods: 23 United States sites including Ambulatory Surgical Center Setting (ASC) and Outpatient Clinical settings. Respondents were physicians who had early experience with DEX in cataract surgery patients. This was a Phase 4 experiential cross-sectional survey study comprised of 3 sequential online physician surveys. Descriptive statistics summarized the surveys' responses to determine the early impressions of the respondents.
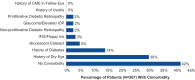
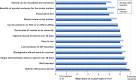
Results: Forty-two physicians completed surveys. On average, physicians reported feeling comfortable administering DEX after placing 3 inserts (mean 2.7; standard deviation 1.9). Most physicians (92%) were satisfied with DEX, and all physicians (100%) reported that DEX improved patient compliance. Most physicians (62.5%) indicated they would highly prefer DEX over traditional steroid eyedrops for the management of post-surgical inflammation and pain.
Conclusion: The surveys exploring the early use of DEX suggest that DEX is a clinically effective treatment with a rapid initial learning curve and integrates well into clinical use. Physicians had a very positive early experience with DEX, including comfort with insertion and satisfaction. DEX shows promise as a primary treatment choice of physicians for ocular inflammation and pain following cataract surgery by offering patients a hands-free innovative therapy that delivers a preservative-free steroid to the ocular surface over approximately 30 days.
Keywords: hands-free therapy; intracanalicular dexamethasone insert; ocular inflammation; ocular pain; phacoemulsification; sustained-release drug delivery.
© 2022 Matossian et al.
Conflict of interest statement
Funding/support provided by Ocular Therapeutix, Inc. Cynthia Matossian, John D Stephens, Steven E Smith, Parag A Majmudar, Subba Rao Gollamudi, Ravi H Patel, and Maria E Rosselson received support from Ocular Therapeutix, Inc. for their participation in the study. Aditi Bauskar, Alyssa Montieth, Fabiana Q Silva, Srilatha Vantipalli, Andrea Gibson, Jamie Lynne Metzinger, and Michael H Goldstein are employees of Ocular Therapeutix, Inc. Michelle K Rhee reports grants from Ocular Therapeutix, on advisory board for NovaBay and Nevakar, outside the submitted work; and Research support from Ocular Therapeutix. The authors report no other conflicts of interest in this work.
Figures


References
-
- Weiner G. Savvy steroid use. Eyenet Web site. Available from: https://www.aao.org/eyenet/article/savvy-steroid-use. Accessed August 10, 2021.
-
- Cataract Surgeons for Improved Eyecare. Analysis of the economic impacts of dropless cataract therapy on Medicare, Medicaid, state governments, and patient costs. Available from: http://stateofreform.com/wp-content/uploads/2015/11/CSIE_Dropless_Econom.... Accessed August. 10, 2021.
-
- Durezol ® (difluprednate ophthalmic emulsion) [package insert]. East Hanover, New Jersey: Novartis Pharmaceuticals; 2020.
LinkOut - more resources
Full Text Sources
Miscellaneous

