Genome-wide identification and functional exploration of the legume lectin genes in Brassica napus and their roles in Sclerotinia disease resistance
- PMID: 35968144
- PMCID: PMC9374194
- DOI: 10.3389/fpls.2022.963263
Genome-wide identification and functional exploration of the legume lectin genes in Brassica napus and their roles in Sclerotinia disease resistance
Abstract
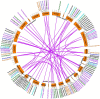
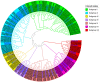
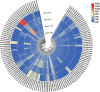
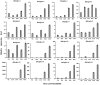
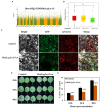
As one of the largest classes of lectins, legume lectins have a variety of desirable features such as antibacterial and insecticidal activities as well as anti-abiotic stress ability. The Sclerotinia disease (SD) caused by the soil-borne fungus Sclerotinia sclerotiorum is a devastating disease affecting most oil crops such as Brassica napus. Here, we identified 130 legume lectin (LegLu) genes in B. napus, which could be phylogenetically classified into seven clusters. The BnLegLu gene family has been significantly expanded since the whole-genome duplication (WGD) or segmental duplication. Gene structure and conserved motif analysis suggested that the BnLegLu genes were well conserved in each cluster. Moreover, relative to those genes only containing the legume lectin domain in cluster VI-VII, the genes in cluster I-V harbored a transmembrane domain and a kinase domain linked to the legume lectin domain in the C terminus. The expression of most BnLegLu genes was relatively low in various tissues. Thirty-five BnLegLu genes were responsive to abiotic stress, and 40 BnLegLu genes were strongly induced by S. sclerotiorum, with a most significant up-regulation of 715-fold, indicating their functional roles in SD resistance. Four BnLegLu genes were located in the candidate regions of genome-wide association analysis (GWAS) results which resulted from a worldwide rapeseed population consisting of 324 accessions associated with SD. Among them, the positive role of BnLegLus-16 in SD resistance was validated by transient expression in tobacco leaves. This study provides important information on BnLegLu genes, particularly about their roles in SD resistance, which may help targeted functional research and genetic improvement in the breeding of B. napus.
Keywords: Brassica napus; Sclerotinia sclerotiorum; genome-wide association study; legume lectin; phylogenetic analysis.
Copyright © 2022 Zuo, Xie, Gao, Liu, Tang, Cheng, Liu, Bai and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
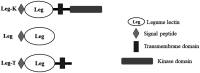

Similar articles
-
The Characterization of the Phloem Protein 2 Gene Family Associated with Resistance to Sclerotinia sclerotiorum in Brassica napus.Int J Mol Sci. 2022 Apr 1;23(7):3934. doi: 10.3390/ijms23073934. Int J Mol Sci. 2022. PMID: 35409295 Free PMC article.
-
Genome-wide identification and functional analysis of cupin_1 domain-containing members involved in the responses to Sclerotinia sclerotiorum and abiotic stress in Brassica napus.Front Plant Sci. 2022 Aug 1;13:983786. doi: 10.3389/fpls.2022.983786. eCollection 2022. Front Plant Sci. 2022. PMID: 35979083 Free PMC article.
-
Genome Structures and Evolution Analysis of Hsp90 Gene Family in Brassica napus Reveal the Possible Roles of Members in Response to Salt Stress and the Infection of Sclerotinia sclerotiorum.Front Plant Sci. 2022 Apr 7;13:854034. doi: 10.3389/fpls.2022.854034. eCollection 2022. Front Plant Sci. 2022. PMID: 35463405 Free PMC article.
-
Functional and evolutionary study of MLO gene family in the regulation of Sclerotinia stem rot resistance in Brassica napus L.Biotechnol Biofuels Bioprod. 2023 May 23;16(1):86. doi: 10.1186/s13068-023-02325-z. Biotechnol Biofuels Bioprod. 2023. PMID: 37217949 Free PMC article.
-
Sclerotinia Stem Rot Resistance in Rapeseed: Recent Progress and Future Prospects.J Agric Food Chem. 2021 Mar 17;69(10):2965-2978. doi: 10.1021/acs.jafc.0c07351. Epub 2021 Mar 5. J Agric Food Chem. 2021. PMID: 33667087 Review.
Cited by
-
Evolution and function of galectins in Xenopus laevis: Comparison with mammals and new perspectives.BBA Adv. 2025 Mar 15;7:100157. doi: 10.1016/j.bbadva.2025.100157. eCollection 2025. BBA Adv. 2025. PMID: 40224191 Free PMC article.
References
-
- Armijo G., Salinas P., Monteoliva M. I., Seguel A., García C., Villarroel-Candia E., et al. . (2013). A salicylic acid-induced lectin-like protein plays a positive role in the effector-triggered immunity response of Arabidopsis thaliana to Pseudomonas syringae Avr-Rpm1. Mol. Plant Microbe Interact. 26, 1395–1406. doi: 10.1094/mpmi-02-13-0044-r, PMID: - DOI - PubMed
-
- Balagué C., Gouget A., Bouchez O., Souriac C., Haget N., Boutet-Mercey S., et al. . (2017). The Arabidopsis thaliana lectin receptor kinase LecRK-I.9 is required for full resistance to pseudomonas syringae and affects jasmonate signalling. Mol. Plant Pathol. 18, 937–948. doi: 10.1111/mpp.12457, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

