Differential regulations of abscisic acid-induced desiccation tolerance and vegetative dormancy by group B3 Raf kinases in liverworts
- PMID: 35968153
- PMCID: PMC9370073
- DOI: 10.3389/fpls.2022.952820
Differential regulations of abscisic acid-induced desiccation tolerance and vegetative dormancy by group B3 Raf kinases in liverworts
Abstract
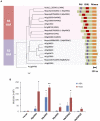
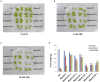
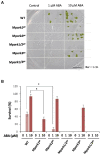
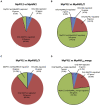
Phytohormone abscisic acid (ABA) plays a key role in stomata closure, osmostress acclimation, and vegetative and embryonic dormancy. Group B3 Raf protein kinases (B3-Rafs) serve as positive regulators of ABA and osmostress signaling in the moss Physcomitrium patens and the angiosperm Arabidopsis thaliana. While P. patens has a single B3-Raf called ARK, specific members of B3-Rafs among six paralogs regulate ABA and osmostress signaling in A. thaliana, indicating functional diversification of B3-Rafs in angiosperms. However, we found that the liverwort Marchantia polymorpha, belonging to another class of bryophytes, has three paralogs of B3-Rafs, MpARK1, MpARK2, and MpARK3, with structural variations in the regulatory domains of the polypeptides. By reporter assays of the P. patens ark line and analysis of genome-editing lines of M. polymorpha, we found that these B3-Rafs are functionally redundant in ABA response, with respect to inhibition of growth, tolerance to desiccation and expression of stress-associated transcripts, the majority of which are under the control of the PYR/PYL/RCAR-like receptor MpPYL1. Interestingly, gemmae in gemma cups were germinating only in mutant lines associated with MpARK1, indicating that dormancy in the gametophyte is controlled by a specific B3-Raf paralog. These results indicated not only conservation of the role of B3-Rafs in ABA and osmostress response in liverworts but also functional diversification of B3-Rafs, which is likely to have occurred in the early stages of land plant evolution.
Keywords: Marchantia polymorpha; RNA-seq; Raf kinase; abscisic acid; genome editing; liverworts; vegetative dormancy.
Copyright © 2022 Jahan, Yamazaki, Islam, Ghosh, Yoshimura, Kato, Ishizaki, Shinozawa, Sakata and Takezawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Archetypal Roles of an Abscisic Acid Receptor in Drought and Sugar Responses in Liverworts.Plant Physiol. 2019 Jan;179(1):317-328. doi: 10.1104/pp.18.00761. Epub 2018 Nov 15. Plant Physiol. 2019. PMID: 30442644 Free PMC article.
-
An Evolutionarily Conserved Abscisic Acid Signaling Pathway Regulates Dormancy in the Liverwort Marchantia polymorpha.Curr Biol. 2018 Nov 19;28(22):3691-3699.e3. doi: 10.1016/j.cub.2018.10.018. Epub 2018 Nov 8. Curr Biol. 2018. PMID: 30416060
-
Arabidopsis Raf-like kinases act as positive regulators of subclass III SnRK2 in osmostress signaling.Plant J. 2020 Jul;103(2):634-644. doi: 10.1111/tpj.14756. Epub 2020 Apr 18. Plant J. 2020. PMID: 32239564 Free PMC article.
-
Decoding ABA and osmostress signalling in plants from an evolutionary point of view.Plant Cell Environ. 2020 Dec;43(12):2894-2911. doi: 10.1111/pce.13869. Epub 2020 Sep 24. Plant Cell Environ. 2020. PMID: 33459424 Review.
-
Gemma cup and gemma development in Marchantia polymorpha.New Phytol. 2020 Oct;228(2):459-465. doi: 10.1111/nph.16655. Epub 2020 Jun 19. New Phytol. 2020. PMID: 32390245 Review.
Cited by
-
Environmental Pollutant Anthracene Induces ABA-Dependent Transgenerational Effects on Gemmae Dormancy in Marchantia polymorpha.Plants (Basel). 2024 Oct 25;13(21):2979. doi: 10.3390/plants13212979. Plants (Basel). 2024. PMID: 39519898 Free PMC article.
References
-
- Akter K., Kato M., Sato Y., Kaneko Y., Takezawa D. (2014). Abscisic acid-induced rearrangement of intracellular structures associated with freezing and desiccation stress tolerance in the liverwort Marchantia polymorpha. J. Plant Physiol. 171, 1334–1343. doi: 10.1016/j.jplph.2014.05.004, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

