Efficacy and safety of acupuncture combined with auricular acupressure for smoking cessation: A study protocol of a multicentre, randomized, controlled clinical trial
- PMID: 35968287
- PMCID: PMC9363779
- DOI: 10.3389/fneur.2022.921054
Efficacy and safety of acupuncture combined with auricular acupressure for smoking cessation: A study protocol of a multicentre, randomized, controlled clinical trial
Abstract
Background: Nicotine dependence is an addictive behavioral disease facilitated by habitually smoking cigarettes. In many countries, acupuncture and auricular acupressure have attracted growing attention as complementary or alternative treatments for smoking cessation; however, there is a lack of rigorous randomized, controlled studies evaluating the combination of these two interventions specifically for smoking cessation. The aim of this study is to evaluate the efficacy and safety of using acupuncture combined with auricular acupressure (A&AA) to increase the rates of smoking cessation and ultimately reduce the rates of relapse.
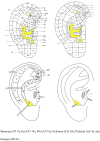
Methods: This is a multicentre, prospective, parallel, randomized, controlled trial. A total of 360 patients with severe nicotine dependence will be randomized into test (A&AA) or control (nicotine replacement therapy, NRT) groups. The test group will be treated with A&AA twice weekly, while the control group will use an NRT patch daily. All treatments will be administered for 8 weeks, with a follow-up period of 4 months. The primary outcome will be the smoking abstinence rate at week 24, with a combined safety assessment. The secondary outcomes will be smoking cessation rates at other timepoints, saliva cortisone test results, and scores on the Fagerstrom Test for Nicotine Dependence, the Autonomy over Tobacco Scale, the Hamilton Anxiety Rating Scale, the Self-rating Anxiety Scale, and the Pittsburgh Sleep Quality Index. The cost of treatment will also be used to evaluate the economic effects of different smoking cessation interventions. Statistical analysis on the data collected from both the intention-to-treat (all randomly assigned patients) and per-protocol (patients who complete the trial without any protocol deviations) patients, will be performed using the statistical software package, IBM SPSS 27.0.
Discussion: This study will provide rigorous clinical evidence evaluating the efficacy and safety of using A&AA as a smoking cessation therapy.
Trial registration: Chinese Clinical Trial Registry (Registration number: ChiCTR1900028371).
Keywords: acupuncture combined with auricular acupressure (A&AA); acupuncture therapy; auricular acupressure therapy; nicotine dependence; smoking cessation.
Copyright © 2022 Zeng, Liao, Wei, Chen, Cai, Chen, Gou and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor CT declared a shared parent affiliation with the authors JZ, YL, XW, GC, ZC, YG, and GL at the time of review.
Figures
Similar articles
-
Acupuncture combined with auricular acupressure for smoking cessation and its effects on tobacco dependence and smoking behavior among Hong Kong smokers: a multicenter pilot clinical study.Chin Med. 2022 Aug 9;17(1):92. doi: 10.1186/s13020-022-00649-w. Chin Med. 2022. PMID: 35941599 Free PMC article.
-
Efficacy and safety evaluation of adjuvant auricular acupuncture for smoking cessation: A study protocol of randomized, assessor-blinded, pragmatic pilot trial.Medicine (Baltimore). 2022 Oct 28;101(43):e31456. doi: 10.1097/MD.0000000000031456. Medicine (Baltimore). 2022. PMID: 36316847 Free PMC article.
-
[Effect of the different smoking cessation regimens with acupuncture on smoking withdrawal and their influence factors: a multi-center randomized controlled trial].Zhongguo Zhen Jiu. 2019 Dec 12;39(12):1255-61. doi: 10.13703/j.0255-2930.2019.12.001. Zhongguo Zhen Jiu. 2019. PMID: 31820598 Clinical Trial. Chinese.
-
The effectiveness and safety of auricular acupoint-related therapy for nicotine dependence: A systematic review and meta-analysis.Tob Induc Dis. 2025 Feb 10;23. doi: 10.18332/tid/200550. eCollection 2025. Tob Induc Dis. 2025. PMID: 39931130 Free PMC article. Review.
-
Association of Acupuncture and Auricular Acupressure With the Improvement of Sleep Disturbances in Cancer Survivors: A Systematic Review and Meta-Analysis.Front Oncol. 2022 May 18;12:856093. doi: 10.3389/fonc.2022.856093. eCollection 2022. Front Oncol. 2022. PMID: 35664757 Free PMC article.
Cited by
-
Silver Spike Point Therapy in smoking cessation: What is it and does it work?Saudi Med J. 2023 Jun;44(6):537-543. doi: 10.15537/smj.2023.44.6.20220848. Saudi Med J. 2023. PMID: 37343991 Free PMC article. Review.
References
-
- WHO . Tobacco Free Initiative. Available online at: http://www.who.int/tobacco/healthpriority/en/ (accessed September 10, 2018).
-
- Yu B, Wang X. Overview of smoking addiction mechanism. Chin J Drug Abuse Prev. (2013) 19:275–8. 10.3969/j.issn.1006-902X.2013.05.009 - DOI
-
- Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. (2010) 8:8. 10.1186/1617-9625-8-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources