Different interventional time of hepatic arterial infusion with PD-1 inhibitor for advanced biliary tract cancer: a multicenter retrospective study
- PMID: 35968352
- PMCID: PMC9360239
Different interventional time of hepatic arterial infusion with PD-1 inhibitor for advanced biliary tract cancer: a multicenter retrospective study
Abstract
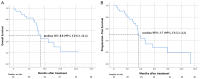
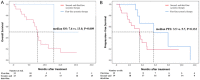
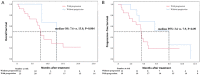
This study investigated the efficacy and safety of hepatic artery infusion chemotherapy (HAIC) combined with PD-1 immunotherapy for advanced biliary tract cancer (BTC) and evaluated the optimal timing of HAIC. A total of 36 unresectable BTC patients treated with HAIC and PD-1 inhibitors between September 2019 and July 2021 were included in this study. Overall survival (OS), progression-free survival (PFS), tumor response, and adverse events (AEs) were investigated. Overall, 52.8% patients with advanced BTC were in stage IV, 23 patients who progressed after receiving PD-1 inhibitor had undergone HAIC, and 23 patients have received 2 or more lines of therapy. The median OS was 8.8 months (range: 4.0-24.0 months), and the median PFS was 3.7 months. The objective response rate and disease control rate were 11.5% and 76.9%, respectively. In the subgroup analysis, patients who treated with HAIC early without progression after immunotherapy were associated with a trend toward better OS (median 13.0 vs. 7.6 months; P = 0.004) and PFS (median 7.9 vs. 3.6 months; P = 0.09) compared to with HAIC with progression after PD-1 treatment. No treatment-related deaths occurred. A total of 44.4% of the patients experienced grade 3 or 4 AEs. We conclude that the combination of HAIC and PD-1 inhibitors is safe and effective. Early HAIC combined with immunotherapy can effectively prolong the overall survival of patients with advanced BTC.
Keywords: Biliary tract cancer; PD-1 inhibitor; combination therapy; hepatic arterial infusion chemotherapy; interventional time of HAIC.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures
References
-
- Banales JM, Marin JJG, Lamarca A, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. - PMC - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
-
- Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019;37:4003–4003.
-
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. - PMC - PubMed
LinkOut - more resources
Full Text Sources
