Histopathological modeling of status epilepticus-induced brain damage based on in vivo diffusion tensor imaging in rats
- PMID: 35968364
- PMCID: PMC9372371
- DOI: 10.3389/fnins.2022.944432
Histopathological modeling of status epilepticus-induced brain damage based on in vivo diffusion tensor imaging in rats
Abstract
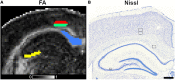
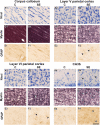
Non-invasive magnetic resonance imaging (MRI) methods have proved useful in the diagnosis and prognosis of neurodegenerative diseases. However, the interpretation of imaging outcomes in terms of tissue pathology is still challenging. This study goes beyond the current interpretation of in vivo diffusion tensor imaging (DTI) by constructing multivariate models of quantitative tissue microstructure in status epilepticus (SE)-induced brain damage. We performed in vivo DTI and histology in rats at 79 days after SE and control animals. The analyses focused on the corpus callosum, hippocampal subfield CA3b, and layers V and VI of the parietal cortex. Comparison between control and SE rats indicated that a combination of microstructural tissue changes occurring after SE, such as cellularity, organization of myelinated axons, and/or morphology of astrocytes, affect DTI parameters. Subsequently, we constructed a multivariate regression model for explaining and predicting histological parameters based on DTI. The model revealed that DTI predicted well the organization of myelinated axons (cross-validated R = 0.876) and astrocyte processes (cross-validated R = 0.909) and possessed a predictive value for cell density (CD) (cross-validated R = 0.489). However, the morphology of astrocytes (cross-validated R > 0.05) was not well predicted. The inclusion of parameters from CA3b was necessary for modeling histopathology. Moreover, the multivariate DTI model explained better histological parameters than any univariate model. In conclusion, we demonstrate that combining several analytical and statistical tools can help interpret imaging outcomes to microstructural tissue changes, opening new avenues to improve the non-invasive diagnosis and prognosis of brain tissue damage.
Keywords: astrocyte morphology; cell counting; diffusion tensor imaging; predictive modeling; structure tensor analysis.
Copyright © 2022 San Martín Molina, Salo, Gröhn, Tohka and Sierra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Diffusion tensor MRI shows progressive changes in the hippocampus and dentate gyrus after status epilepticus in rat - histological validation with Fourier-based analysis.Neuroimage. 2017 May 15;152:221-236. doi: 10.1016/j.neuroimage.2017.03.003. Epub 2017 Mar 4. Neuroimage. 2017. PMID: 28267625
-
In Vivo Diffusion Tensor Imaging in Acute and Subacute Phases of Mild Traumatic Brain Injury in Rats.eNeuro. 2020 Jun 15;7(3):ENEURO.0476-19.2020. doi: 10.1523/ENEURO.0476-19.2020. Print 2020 May/Jun. eNeuro. 2020. PMID: 32424056 Free PMC article.
-
Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology.Neuroimage. 2014 Aug 15;97:363-73. doi: 10.1016/j.neuroimage.2014.04.013. Epub 2014 Apr 15. Neuroimage. 2014. PMID: 24742916
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
-
The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter.MAGMA. 2013 Aug;26(4):345-70. doi: 10.1007/s10334-013-0371-x. Epub 2013 Feb 27. MAGMA. 2013. PMID: 23443883 Free PMC article. Review.
Cited by
-
A multiscale tissue assessment in a rat model of mild traumatic brain injury.J Neuropathol Exp Neurol. 2022 Dec 19;82(1):71-83. doi: 10.1093/jnen/nlac100. J Neuropathol Exp Neurol. 2022. PMID: 36331507 Free PMC article.
References
LinkOut - more resources
Full Text Sources

