Modulation of entorhinal cortex-hippocampus connectivity and recognition memory following electroacupuncture on 3×Tg-AD model: Evidence from multimodal MRI and electrophysiological recordings
- PMID: 35968386
- PMCID: PMC9372370
- DOI: 10.3389/fnins.2022.968767
Modulation of entorhinal cortex-hippocampus connectivity and recognition memory following electroacupuncture on 3×Tg-AD model: Evidence from multimodal MRI and electrophysiological recordings
Abstract
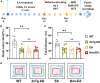
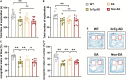
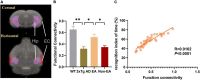
Memory loss and aberrant neuronal network activity are part of the earliest hallmarks of Alzheimer's disease (AD). Electroacupuncture (EA) has been recognized as a cognitive stimulation for its effects on memory disorder, but whether different brain regions or neural circuits contribute to memory recovery in AD remains unknown. Here, we found that memory deficit was ameliorated in 3×Tg-AD mice with EA-treatment, as shown by the increased number of exploring and time spent in the novel object. In addition, reduced locomotor activity was observed in 3×Tg-AD mice, but no significant alteration was seen in the EA-treated mice. Based on the functional magnetic resonance imaging, the regional spontaneous activity alterations of 3×Tg-AD were mainly concentrated in the accumbens nucleus, auditory cortex, caudate putamen, entorhinal cortex (EC), hippocampus, insular cortex, subiculum, temporal cortex, visual cortex, and so on. While EA-treatment prevented the chaos of brain activity in parts of the above regions, such as the auditory cortex, EC, hippocampus, subiculum, and temporal cortex. And then we used the whole-cell voltage-clamp recording to reveal the neurotransmission in the hippocampus, and found that EA-treatment reversed the synaptic spontaneous release. Since the hippocampus receives most of the projections of the EC, the hippocampus-EC circuit is one of the neural circuits related to memory impairment. We further applied diffusion tensor imaging (DTI) tracking and functional connectivity, and found that hypo-connected between the hippocampus and EC with EA-treatment. These data indicate that the hippocampus-EC connectivity is responsible for the recognition memory deficit in the AD mice with EA-treatment, and provide novel insight into potential therapies for memory loss in AD.
Keywords: Alzheimer’s disease; electroacupuncture; electrophysiology; functional connectivity; recognition memory.
Copyright © 2022 Lin, Zhang, Yin, Chen, Ruan, Wu, Liu and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

