Stress Affects Central Compensation of Neural Responses to Cochlear Synaptopathy in a cGMP-Dependent Way
- PMID: 35968392
- PMCID: PMC9372611
- DOI: 10.3389/fnins.2022.864706
Stress Affects Central Compensation of Neural Responses to Cochlear Synaptopathy in a cGMP-Dependent Way
Abstract
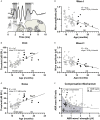
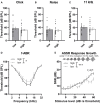
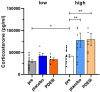
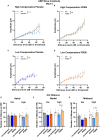
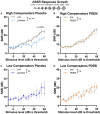
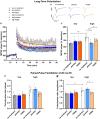
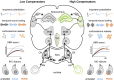
In light of the increasing evidence supporting a link between hearing loss and dementia, it is critical to gain a better understanding of the nature of this relationship. We have previously observed that following cochlear synaptopathy, the temporal auditory processing (e.g., auditory steady state responses, ASSRs), is sustained when reduced auditory input is centrally compensated. This central compensation process was linked to elevated hippocampal long-term potentiation (LTP). We further observed that, independently of age, central responsiveness to cochlear synaptopathy can differ, resulting in either a low or high capacity to compensate for the reduced auditory input. Lower central compensation resulted in poorer temporal auditory processing, reduced hippocampal LTP, and decreased recruitment of activity-dependent brain-derived neurotrophic factor (BDNF) expression in hippocampal regions (low compensators). Higher central compensation capacity resulted in better temporal auditory processing, higher LTP responses, and increased activity-dependent BDNF expression in hippocampal regions. Here, we aimed to identify modifying factors that are potentially responsible for these different central responses. Strikingly, a poorer central compensation capacity was linked to lower corticosterone levels in comparison to those of high compensators. High compensators responded to repeated placebo injections with elevated blood corticosterone levels, reduced auditory brainstem response (ABR) wave I amplitude, reduced inner hair cell (IHC) ribbon number, diminished temporal processing, reduced LTP responses, and decreased activity-dependent hippocampal BDNF expression. In contrast, the same stress exposure through injection did not elevate blood corticosterone levels in low compensators, nor did it reduce IHC ribbons, ABR wave I amplitude, ASSR, LTP, or BDNF expression as seen in high compensators. Interestingly, in high compensators, the stress-induced responses, such as a decline in ABR wave I amplitude, ASSR, LTP, and BDNF could be restored through the "memory-enhancing" drug phosphodiesterase 9A inhibitor (PDE9i). In contrast, the same treatment did not improve these aspects in low compensators. Thus, central compensation of age-dependent cochlear synaptopathy is a glucocorticoid and cyclic guanosine-monophosphate (cGMP)-dependent neuronal mechanism that fails upon a blunted stress response.
Keywords: blunted stress response; cGMP; cochlear synaptopathy; glucocorticoid; long-term potentiation; phosphodiesterase 9A inhibitor (PDE9i).
Copyright © 2022 Savitska, Hess, Calis, Marchetta, Harasztosi, Fink, Eckert, Ruth, Rüttiger, Knipper and Singer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor VS declared a past collaboration with the authors, LR, MK, and WS.
Figures

Similar articles
-
Age-Dependent Auditory Processing Deficits after Cochlear Synaptopathy Depend on Auditory Nerve Latency and the Ability of the Brain to Recruit LTP/BDNF.Brain Sci. 2020 Oct 6;10(10):710. doi: 10.3390/brainsci10100710. Brain Sci. 2020. PMID: 33036168 Free PMC article.
-
Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility.Hear Res. 2018 Aug;365:36-48. doi: 10.1016/j.heares.2018.06.003. Epub 2018 Jun 12. Hear Res. 2018. PMID: 29913342
-
Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain.Neurobiol Aging. 2016 Aug;44:173-184. doi: 10.1016/j.neurobiolaging.2016.05.001. Epub 2016 May 10. Neurobiol Aging. 2016. PMID: 27318145
-
Detecting Noise-Induced Cochlear Synaptopathy by Auditory Brainstem Response in Tinnitus Patients With Normal Hearing Thresholds: A Meta-Analysis.Front Neurosci. 2021 Dec 20;15:778197. doi: 10.3389/fnins.2021.778197. eCollection 2021. Front Neurosci. 2021. PMID: 34987358 Free PMC article.
-
Use of the auditory brainstem response for assessment of cochlear synaptopathy in humans.J Acoust Soc Am. 2021 Dec;150(6):4440. doi: 10.1121/10.0007484. J Acoust Soc Am. 2021. PMID: 34972291 Free PMC article. Review.
Cited by
-
The 10th International Conference on cGMP 2022: recent trends in cGMP research and development-meeting report.Naunyn Schmiedebergs Arch Pharmacol. 2023 Aug;396(8):1669-1686. doi: 10.1007/s00210-023-02484-8. Epub 2023 Apr 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37079081 Free PMC article. Review.
-
Acute deletion of the central MR/GR steroid receptor correlates with changes in LTP, auditory neural gain, and GC-A cGMP signaling.Front Mol Neurosci. 2023 Feb 17;16:1017761. doi: 10.3389/fnmol.2023.1017761. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36873102 Free PMC article.
References
-
- Arango-Lievano M., Borie A. M., Dromard Y., Murat M., Desarmenien M. G., Garabedian M. J., et al. . (2019). Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors. Proc. Natl. Acad. Sci. U. S. A. 116, 13097–13106. 10.1073/pnas.1903203116 - DOI - PMC - PubMed
-
- Burkard R. F., Don M. (2007). “The auditory brainstem response,” in Auditory Evoked Potentials: Basic Principles and Clinical Application, eds. R.F. Burkard, J.J. Eggermont, and M. Don. (Philadelphia: Lippincott Williams and Wilkins; ).
LinkOut - more resources
Full Text Sources

