SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling
- PMID: 35968603
- PMCID: PMC9633309
- DOI: 10.1111/cas.15531
SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling
Abstract
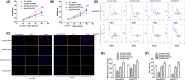
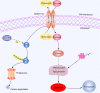
SHARPIN is a tumor-associated gene involved in the growth and proliferation of many tumor types. A function of SHARPIN in cholangiocarcinoma (CCA) is so far unclear. Here, we studied the role and function of SHARPIN in CCA and revealed its relevant molecular mechanism. The expression of SHARPIN was analyzed in cholangiocarcinoma tissues from patients using immunohistochemistry, quantitative PCR, and western blot analysis. Expression of SHARPIN was suppressed/overexpressed by siRNA silencing or lentiviral overexpression vector, and the effect on cell proliferation was determined by the CCK-8 assay and flow cytometry. Accumulation of reactive oxygen species was measured with MitoTracker, and JC-1 staining showed mitochondrial fission/fusion and mitochondrial membrane potential changes as a result of the silencing or overexpression. The ferroptosis marker solute carrier family 7 member 11 (SLC7A11), glutathione peroxidase 4 (GPX4), and the antioxidant enzymes superoxide dismutase 1 (SOD-1) and SOD-2 were analyzed by western blot. The results showed that SHARPIN expression was increased in CCA tissue, and this was involved in cell proliferation. SHARPIN silencing resulted in accumulated reactive oxygen species, reduced mitochondrial fission, and a reduced mitochondrial membrane potential. Silencing of SHARPIN inhibited the ubiquitination and degradation of p53, and downregulated levels of SLC7A11, GPX4, SOD-1, and SOD-2, all of which contributed to excessive oxidative stress that leads to ferroptosis. Overexpression of SHARPIN would reverse the above process. The collected data suggest that in CCA, SHARPIN-mediated cell ferroptosis via the p53/SLC7A11/GPX4 signaling pathway is inhibited. Targeting SHARPIN might be a promising approach for the treatment of CCA.
Keywords: SHARPIIN; cholangiocarcinoma; ferroptosis; mitochondria; proliferation.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures




References
-
- Lendvai G, Szekerczes T, Illyes I, et al. Cholangiocarcinoma: classification, histopathology and molecular carcinogenesis. Pathol Oncol Res. 2020;26(1):3‐15. - PubMed
-
- Seymour RE, Hasham MG, Cox GA, et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8(5):416‐421. - PubMed
MeSH terms
Substances
Grants and funding
- 2021A1515010928/Natural Scientific Foundation of Guangdong Province
- SRSP2019012/Scientific ResearchStar plan of Shunde Hospital
- SRSP2021003/Scientific ResearchStar plan of Shunde Hospital
- SRSP2021016/Scientific ResearchStar plan of Shunde Hospital
- SRSP2021005/Scientific ResearchStar plan of Shunde Hospital
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

