Targeting SIRT1 Rescues Age- and Obesity-Induced Microvascular Dysfunction in Ex Vivo Human Vessels
- PMID: 35968712
- PMCID: PMC9426744
- DOI: 10.1161/CIRCRESAHA.122.320888
Targeting SIRT1 Rescues Age- and Obesity-Induced Microvascular Dysfunction in Ex Vivo Human Vessels
Abstract
Background: Experimental evidence suggests a key role of SIRT1 (silent information regulator 1) in age- and metabolic-related vascular dysfunction. Whether these effects hold true in the human microvasculature is unknown. We aimed to investigate the SIRT1 role in very early stages of age- and obesity-related microvascular dysfunction in humans.
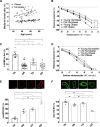
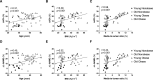
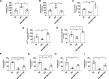
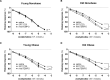
Methods: Ninety-five subjects undergoing elective laparoscopic surgery were recruited and stratified based on their body mass index status (above or below 30 kg/m2) and age (above or below 40 years) in 4 groups: Young Nonobese, Young Obese, Old Nonobese, and Old Obese. We measured small resistance arteries' endothelial function by pressurized micromyography before and after incubation with a SIRT1 agonist (SRT1720) and a mitochondria reactive oxygen species (mtROS) scavenger (MitoTEMPO). We assessed vascular levels of mtROS and nitric oxide availability by confocal microscopy and vascular gene expression of SIRT1 and mitochondrial proteins by qPCR. Chromatin immunoprecipitation assay was employed to investigate SIRT1-dependent epigenetic regulation of mitochondrial proteins.
Results: Compared with Young Nonobese, obese and older patients showed lower vascular expression of SIRT1 and antioxidant proteins (FOXO3 [forkhead box protein O3] and SOD2) and higher expression of pro-oxidant and aging mitochondria proteins p66Shc and Arginase II. Old Obese, Young Obese and Old Nonobese groups endothelial dysfunction was rescued by SRT1720. The restoration was comparable to the one obtained with mitoTEMPO. These effects were explained by SIRT1-dependent chromatin changes leading to reduced p66Shc expression and upregulation of proteins involved in mitochondria respiratory chain.
Conclusions: SIRT1 is a novel central modulator of the earliest microvascular damage induced by age and obesity. Through a complex epigenetic control mainly involving p66Shc and Arginase II, it influences mtROS levels, NO availability, and the expression of proteins of the mitochondria respiratory chain. Therapeutic modulation of SIRT1 restores obesity- and age-related endothelial dysfunction. Early targeting of SIRT1 might represent a crucial strategy to prevent age- and obesity-related microvascular dysfunction.
Keywords: aging; endothelial cells; microcirculation; mitochondria; obesity; sirtuins.
Figures



References
-
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4 - PubMed
-
- Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301 - PubMed
-
- Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–724. doi: 10.1016/S2213-8587(18)30137-2 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

