Upregulated PD-1 signaling antagonizes glomerular health in aged kidneys and disease
- PMID: 35968783
- PMCID: PMC9374384
- DOI: 10.1172/JCI156250
Upregulated PD-1 signaling antagonizes glomerular health in aged kidneys and disease
Abstract
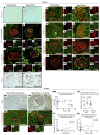
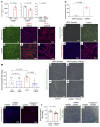
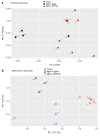
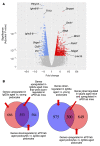
With an aging population, kidney health becomes an important medical and socioeconomic factor. Kidney aging mechanisms are not well understood. We previously showed that podocytes isolated from aged mice exhibit increased expression of programmed cell death protein 1 (PD-1) surface receptor and its 2 ligands (PD-L1 and PD-L2). PDCD1 transcript increased with age in microdissected human glomeruli, which correlated with lower estimated glomerular filtration rate and higher segmental glomerulosclerosis and vascular arterial intima-to-lumen ratio. In vitro studies in podocytes demonstrated a critical role for PD-1 signaling in cell survival and in the induction of a senescence-associated secretory phenotype. To prove PD-1 signaling was critical to podocyte aging, aged mice were injected with anti-PD-1 antibody. Treatment significantly improved the aging phenotype in both kidney and liver. In the glomerulus, it increased the life span of podocytes, but not that of parietal epithelial, mesangial, or endothelial cells. Transcriptomic and immunohistochemistry studies demonstrated that anti-PD-1 antibody treatment improved the health span of podocytes. Administering the same anti-PD-1 antibody to young mice with experimental focal segmental glomerulosclerosis (FSGS) lowered proteinuria and improved podocyte number. These results suggest a critical contribution of increased PD-1 signaling toward both kidney and liver aging and in FSGS.
Keywords: Aging; Chronic kidney disease; Expression profiling.
Figures




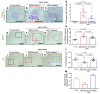
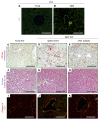
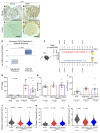
Comment in
-
Aged glomeruli: a link between PD-1 and podocytes.J Clin Invest. 2022 Aug 15;132(16):e162330. doi: 10.1172/JCI162330. J Clin Invest. 2022. PMID: 35968780 Free PMC article.
-
Does treating with anti-PD-1 to improve glomerular health come without a cost? Reply.J Clin Invest. 2022 Nov 15;132(22):e165287. doi: 10.1172/JCI165287. J Clin Invest. 2022. PMID: 36136431 Free PMC article. No abstract available.
-
Does treating with anti-PD-1 to improve glomerular health come without a cost?J Clin Invest. 2022 Nov 15;132(22):e164747. doi: 10.1172/JCI164747. J Clin Invest. 2022. PMID: 36377662 Free PMC article. No abstract available.
-
PD-1 inhibition in aged podocytes and glomerular disease.Kidney Int. 2023 Jan;103(1):18-20. doi: 10.1016/j.kint.2022.11.005. Kidney Int. 2023. PMID: 36603968 No abstract available.
References
-
- Hoy WE, et al. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003;(83):31–37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

