Vaginal bacterium Prevotella timonensis turns protective Langerhans cells into HIV-1 reservoirs for virus dissemination
- PMID: 35968812
- PMCID: PMC9531304
- DOI: 10.15252/embj.2022110629
Vaginal bacterium Prevotella timonensis turns protective Langerhans cells into HIV-1 reservoirs for virus dissemination
Abstract
Dysbiosis of vaginal microbiota is associated with increased HIV-1 acquisition, but the underlying cellular mechanisms remain unclear. Vaginal Langerhans cells (LCs) protect against mucosal HIV-1 infection via autophagy-mediated degradation of HIV-1. As LCs are in continuous contact with bacterial members of the vaginal microbiome, we investigated the impact of commensal and dysbiosis-associated vaginal (an)aerobic bacterial species on the antiviral function of LCs. Most of the tested bacteria did not affect the HIV-1 restrictive function of LCs. However, Prevotella timonensis induced a vast uptake of HIV-1 by vaginal LCs. Internalized virus remained infectious for days and uptake was unaffected by antiretroviral drugs. P. timonensis-exposed LCs efficiently transmitted HIV-1 to target cells both in vitro and ex vivo. Additionally, P. timonensis exposure enhanced uptake and transmission of the HIV-1 variants that establish infection after sexual transmission, the so-called Transmitted Founder variants. Our findings, therefore, suggest that P. timonensis might set the stage for enhanced HIV-1 susceptibility during vaginal dysbiosis and advocate targeted treatment of P. timonensis during bacterial vaginosis to limit HIV-1 infection.
Keywords: HIV-1; Langerhans cells (LCs); Prevotella timonensis; transmission; vaginal microbiome.
© 2022 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
Figures
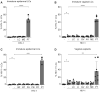
- A, B
Isolated epidermal LCs (A, n = 4) or isolated vaginal LCs (B, n = 5) were stimulated O/N with a variety of vaginal microbiota and subsequently exposed to HIV‐1 (SF162; MOI 0.5). Epidermal LCs were stimulated with L. crispatus (LC), M. elsdenii (ME), and P. timonensis (PT), whereas vaginal LCs were additionally stimulated with L. iners (LI), G. vaginalis (GV), and A. vaginae (AV). After 5 days, HIV‐1 p24 in LCs was determined by an intracellular staining for CD1a and p24 by flow cytometry.
- C
Isolated epidermal LCs (n = 3) were stimulated O/N with B. fragilis (BF), B. thetaiotaomicron (BT), P. amnii (PA), P. bivia (PB), P. copri (PC), and P. timonensis (PT), followed by HIV‐1 (SF162; MOI 0.5) infection for 5 days and HIV‐1 p24 determination by flow cytometry.
- D
Vaginal epithelium explants (n = 8) were exposed to vaginal microbiota as described for isolated vaginal LCs. HIV‐1 p24 in emigrated LCs was determined by flow cytometry.

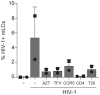
- A–D
Isolated epidermal LCs (A, n = 2), isolated vaginal LCs (B and C, both n = 3), or vaginal explants (D, n = 5) were stimulated O/N with P. timonensis (PT) and exposed to HIV‐1 (SF162; MOI 0.5) for 5 days in the presence or absence of HIV‐1 replication inhibitors (Inh) zidovudine (AZT), tenofovir (TFV), lamivudine (3TC), or indinavir (IDV). HIV‐1 levels were determined by intracellular staining for HIV‐1 p24 using flow cytometry.
- E, F
Representative plots (E) and pooled data (F, n = 3) of HIV‐1 (NL4.3eGFP‐Bal) infection of O/N P. timonensis‐stimulated (PT) isolated vaginal LCs as determined by both GFP‐detection (de novo replication) and HIV‐1 p24 (detection of de novo replication and uptake) by flow cytometry.
- G
Isolated epidermal LCs (n = 3) were stimulated O/N with P. timonensis (PT). Next, LCs were treated with T20, CCR5 inhibitor Maraviroc (CCR5), neutralizing antibodies against CD4 (CD4) and isotype control (Iso), subsequently followed by HIV‐1 exposure for 5 days (SF162; MOI 0.5). Intracellular HIV‐1 p24 levels were determined using flow cytometry.

- A–H
Representative plots of vaginal (A) or epidermal (B) donors and combined experiments (MFI expression relative to untreated condition) of CD80 (C, n = 3), CD86 (D, n = 3), and CCR7 (E, n = 3) surface expression are shown. Ex vivo skin explants were stimulated O/N with L. crispatus (LC), M. elsdenii (ME), P. timonensis (PT) or poly(I:C) and at day 3 post‐inoculation, emigrated LCs were collected and washed and activation phenotype was determined by flow cytometry. Cells were analyzed for CD1a expression, and the absolute number of CD1a‐positive cells, that is LCs, migrated from the epidermis was determined using counting beads; the graph shows the migration relative to the untreated condition (F, n = 5). CD1a‐positive cells, that is LCs, were analyzed using flow cytometry, and the graphs show combined experiments (MFI expression relative to untreated condition) of CD80 (G, n = 5) and CD86 (H, n = 5) surface expression.

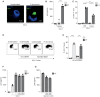
- A
Intracellular HIV‐1 detection was determined by confocal microscopy (Hoechst in blue, HIV‐1 p24 in green); scale bar represents 5 μm (representative donor).
- B
Isolated epidermal LCs were stimulated O/N with P. timonensis (PT) followed by a 4‐h exposure to HIV‐1. HIV‐1 p24 was determined by ELISA after trypsin treatment and lysis of LCs (n = 3).
- C
Isolated epidermal LCs were stimulated O/N with P. timonensis (PT) followed by HIV‐1 (SF162; MOI 0.5) exposure for 3 days. After 3 days, LCs were treated with PBS or trypsin and HIV‐1 p24 was determined by flow cytometry (n = 3).
- D, E
Isolated vaginal LCs were stimulated O/N with P. timonensis (PT) followed by infection with VSV‐g‐BlaM‐Vpr fusion (positive control) and NL4.3Bal‐BlaM‐Vpr fusion. The figure shows representative plots (D) and pooled data (E, n = 3) of viral fusion as determined by β‐lactamase‐Vpr (BlaM‐Vpr) activity.
- F
Isolated epidermal LCs were stimulated O/N with P. timonensis (PT) followed by HIV‐1 exposure (SF162; MOI 0.5) in the presence or absence of anti‐langerin (10E2) or mannan (MAN). HIV‐1 p24 content was determined by ELISA after trypsin treatment and subsequent lysis of LCs (n = 2).
- G
Isolated epidermal LCs were stimulated O/N with P. timonensis (PT) followed by treatment with Rapamycin (μM) and exposure to HIV‐1 (SF162). After 3 days, HIV‐1 p24 was measured by ELISA after trypsin treatment and lysis of LCs (n = 2).

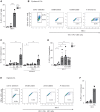
- A
Isolated epidermal LCs were stimulated O/N with P. timonensis (PT) followed by HIV‐1 (SF162; MOI 0.5) exposure. LCs were subjected to repetitive freeze–thaw cycles, and cell lysates were added to U87.CD4.CCR5 cell line. HIV‐1 infection of U87.CD4.CCR5 was determined by an intracellular staining for p24 by flow cytometry (n = 3).
- B, C
Ex vivo skin explants were stimulated O/N with L. crispatus (LC), M. elsdenii (ME), or P. timonensis (PT) and inoculated with HIV‐1 (JRCSF; MOI 0.5) in the presence or absence of tenofovir. At day 2 post‐inoculation, emigrated LCs were collected, washed, and co‐cultured with U87.CD4.CCR5 cells for 3 days. Cells were analyzed for CD1a expression and p24 content by flow cytometry and here presented as representative plots (B) and pooled data of HIV‐1‐positive cells (%) of the CD1a‐negative U87.CD4.CCR5 cells (C, n = 4).
- D
Ex vivo skin explants were stimulated with P. timonensis (PT), pre‐treated with T20 or maraviroc (MAR), and exposed to HIV‐1 (JRCSF; MOI 0.5) for 2 days, after which the migratory fraction was co‐cultured with U87.CD4.CCR5 cells. HIV‐1‐positive cells (%) of the CD1a‐negative fraction, that is, U87.CD4.CCR5 cells, were determined using flow cytometry (n = 5).
- E, F
Isolated vaginal LCs were stimulated O/N with P. timonensis (PT) and infected with HIV‐1 (SF162; MOI 0.5). After 3 days, LCs were washed and co‐cultured with U87.CD4.CCR5. Infection was analyzed by flow cytometry and here shown as representative plots (E) and pooled data of HIV‐1‐positive cells (%) of the CD1a‐negative U87.CD4.CCR5 cells (F, n = 2).
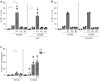
- A, B
Isolated epidermal LCs were stimulated by different Prevotella spp. and Bacteroides spp. followed by T/F variant (CH058; MOI 0.25) exposure in the presence or absence of AZT (A, n = 3) or tenofovir (B, n = 2). A lower MOI was used due to higher susceptibility of LCs for CH058 than for laboratory strains.
- C
HIV‐1 uptake was determined by flow cytometry. Ex vivo skin explants (n = 3) were stimulated O/N with P. timonensis (PT) followed by pre‐treatment with tenofovir (TFV). After pre‐treatment, skin explants were inoculated with lab‐adapted strain (SF162; MOI 0.25) or HIV‐1 T/F strain (CHO58; MOI 0.25). After 2 days, the migratory fraction was collected and co‐cultured with U87.CD4.CCR5 cells. Cells were analyzed for CD1a expression and p24 content in U87.CD4.CCR5 cells by flow cytometry. A lower MOI was used due to higher susceptibility of LCs for CH058 than for laboratory strain SF162.
Similar articles
-
Vaginal Prevotella timonensis Bacteria Enhance HIV-1 Uptake and Differentially Affect Transmission by Distinct Primary Dendritic Cell Subsets.Eur J Immunol. 2025 Mar;55(3):e202451192. doi: 10.1002/eji.202451192. Eur J Immunol. 2025. PMID: 40071689 Free PMC article.
-
Prevotella timonensis Bacteria Associated With Vaginal Dysbiosis Enhance Human Immunodeficiency Virus Type 1 Susceptibility Of Vaginal CD4+ T Cells.J Infect Dis. 2024 Jul 25;230(1):e43-e47. doi: 10.1093/infdis/jiae166. J Infect Dis. 2024. PMID: 39052703 Free PMC article.
-
Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells.J Reprod Immunol. 2020 Apr;138:103085. doi: 10.1016/j.jri.2020.103085. Epub 2020 Jan 22. J Reprod Immunol. 2020. PMID: 32004804
-
Human immunodeficiency virus-1 acquisition in genital mucosa: Langerhans cells as key-players.J Intern Med. 2009 Jan;265(1):18-28. doi: 10.1111/j.1365-2796.2008.02046.x. J Intern Med. 2009. PMID: 19093957 Review.
-
Langerhans cells in innate defense against pathogens.Trends Immunol. 2010 Dec;31(12):452-9. doi: 10.1016/j.it.2010.08.002. Epub 2010 Oct 26. Trends Immunol. 2010. PMID: 21030306 Review.
Cited by
-
Vaginal Prevotella timonensis Bacteria Enhance HIV-1 Uptake and Differentially Affect Transmission by Distinct Primary Dendritic Cell Subsets.Eur J Immunol. 2025 Mar;55(3):e202451192. doi: 10.1002/eji.202451192. Eur J Immunol. 2025. PMID: 40071689 Free PMC article.
-
Social, microbial, and immune factors linking bacterial vaginosis and infectious diseases.J Clin Invest. 2025 Jun 2;135(11):e184322. doi: 10.1172/JCI184322. eCollection 2025 Jun 2. J Clin Invest. 2025. PMID: 40454473 Free PMC article. Review.
-
Prevotella timonensis degrades the vaginal epithelial glycocalyx through high fucosidase and sialidase activities.mBio. 2024 Sep 11;15(9):e0069124. doi: 10.1128/mbio.00691-24. Epub 2024 Aug 20. mBio. 2024. PMID: 39162399 Free PMC article.
-
Prevotella timonensis Bacteria Associated With Vaginal Dysbiosis Enhance Human Immunodeficiency Virus Type 1 Susceptibility Of Vaginal CD4+ T Cells.J Infect Dis. 2024 Jul 25;230(1):e43-e47. doi: 10.1093/infdis/jiae166. J Infect Dis. 2024. PMID: 39052703 Free PMC article.
-
Exploring potential associations between the human microbiota and reservoir of latent HIV.Retrovirology. 2024 Nov 29;21(1):21. doi: 10.1186/s12977-024-00655-w. Retrovirology. 2024. PMID: 39614246 Free PMC article. Review.
References
-
- Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, Cone RA, Tachedjian G (2015) Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 6: 164 - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

