Extrapolation of Adult Efficacy Data to Pediatric Systemic Lupus Erythematosus: Evaluating Similarities in Exposure-Response
- PMID: 35968821
- PMCID: PMC9771895
- DOI: 10.1002/jcph.2139
Extrapolation of Adult Efficacy Data to Pediatric Systemic Lupus Erythematosus: Evaluating Similarities in Exposure-Response
Abstract
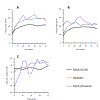
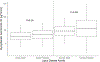
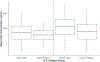
To streamline drug development, the United States Food and Drug Administration (FDA) can consider the extrapolation of adult efficacy data to children when the disease and drug effects are sufficiently similar. This study explored whether the relationship between drug exposure and response for selected drugs in systemic lupus erythematosus (SLE) was sufficiently similar to support a consideration of the extrapolation of adult efficacy data to children of ≥5 years of age. An exposure-response analysis of drugs used to treat SLE was conducted using published exposure versus response and efficacy versus time data. Statistical analyses included noncompartmental analysis of a drug's area under the effect curve and direct Imax pharmacodynamic (PD) modeling. Six drugs were included: azathioprine, belimumab, cyclophosphamide, hydroxychloroquine, mycophenolate/mycophenolic acid, and rituximab. For belimumab, the net change in responders at week 52 (the primary end point) was nearly identical between 1 adult trial and the pediatric trial. For mycophenolate, PD modeling suggested no significant differences in exposure and SLE disease activity between adults and children. For azathioprine, cyclophosphamide, hydroxychloroquine, and rituximab the data were not sufficient to quantitatively characterize the exposure-response relationship, but the clinical or pharmacologic response between children and adults was similar overall. Adult SLE data should be leveraged to guide pediatric drug development programs and identify areas with residual uncertainty regarding the effectiveness or safety of a drug in children. The degree to which efficacy extrapolation can reduce clinical trial requirements in pediatric SLE should be individualized for each new drug product, depending in part on the mechanism of action of the drug and the similarity of disease manifestations in children and adults.
Keywords: pediatrics; pharmacodynamics; pharmacokinetics and drug metabolism; regulatory/scientific affairs; rheumatology.
© 2022, The American College of Clinical Pharmacology.
Conflict of interest statement
Figures

Similar articles
-
[Treatment of systemic lupus erythematosus: myths, certainties and doubts].Med Clin (Barc). 2013 Dec 21;141(12):533-42. doi: 10.1016/j.medcli.2013.02.014. Epub 2013 Apr 23. Med Clin (Barc). 2013. PMID: 23622892 Review. Spanish.
-
EULAR recommendations for the management of systemic lupus erythematosus: 2023 update.Ann Rheum Dis. 2024 Jan 2;83(1):15-29. doi: 10.1136/ard-2023-224762. Ann Rheum Dis. 2024. PMID: 37827694
-
New onset of lupus nephritis in two patients with SLE shortly after initiation of treatment with belimumab.Semin Arthritis Rheum. 2017 Jun;46(6):788-790. doi: 10.1016/j.semarthrit.2016.09.006. Epub 2016 Sep 28. Semin Arthritis Rheum. 2017. PMID: 27793432
-
Transcriptome analysis to decipher the molecular underpinnings of response to treatment in systemic lupus erythematosus.RMD Open. 2025 Mar 12;11(1):e005050. doi: 10.1136/rmdopen-2024-005050. RMD Open. 2025. PMID: 40081913 Free PMC article.
-
Optimizing the use of existing therapies in lupus.Int J Rheum Dis. 2015 Feb;18(2):129-37. doi: 10.1111/1756-185X.12551. Int J Rheum Dis. 2015. PMID: 25884457 Review.
Cited by
-
Extrapolation of the Efficacy and Pharmacokinetics of Belimumab to Support its Use in Children with Lupus Nephritis.Clin Pharmacokinet. 2024 Sep;63(9):1313-1326. doi: 10.1007/s40262-024-01422-y. Epub 2024 Sep 25. Clin Pharmacokinet. 2024. PMID: 39320441 Free PMC article.
-
Achieving big with small: quantitative clinical pharmacology tools for drug development in pediatric rare diseases.J Pharmacokinet Pharmacodyn. 2023 Dec;50(6):429-444. doi: 10.1007/s10928-023-09863-x. Epub 2023 May 4. J Pharmacokinet Pharmacodyn. 2023. PMID: 37140724 Review.
-
CAR T cell therapy for children with rheumatic disease: the time is now.Nat Rev Rheumatol. 2025 Aug;21(8):494-506. doi: 10.1038/s41584-025-01272-3. Epub 2025 Jul 2. Nat Rev Rheumatol. 2025. PMID: 40603629 Review.
-
CAR T cells in pediatric systemic lupus erythematosus.Clin Rheumatol. 2025 Jul 11. doi: 10.1007/s10067-025-07550-5. Online ahead of print. Clin Rheumatol. 2025. PMID: 40646368 Review.
-
New medicines for childhood-onset systemic lupus erythematosus: an EU perspective on paediatric drug development.Front Med (Lausanne). 2025 Jul 10;12:1583140. doi: 10.3389/fmed.2025.1583140. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40708640 Free PMC article.
References
-
- Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128(5):e1242–1249. - PubMed
-
- Product Label. Belimumab injection. GlaxoSmithKline LP, PA. March 11, 2021.
-
- Livingston B, Bonner A, Pope J. Differences in clinical manifestations between childhood-onset lupus and adult-onset lupus: a meta-analysis. Lupus. 2011;20(13):1345–1355. - PubMed
-
- United States Food and Drug Administration (FDA). Pediatric science and research activities. FDA web site. https://www.fda.gov/science-research/pediatrics/pediatric-science-and-re.... Updated October 15, 2021. Accessed October 25, 2021.
-
- United States Food and Drug Administration (FDA). Guidance document: general clinical pharmacology considerations for pediatric studies for drugs and biological products. December 2014. FDA web site. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat.... Accessed October 25, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

