Extrapolation of Adult Efficacy Data to Pediatric Systemic Lupus Erythematosus: Evaluating Similarities in Exposure-Response
- PMID: 35968821
- PMCID: PMC9771895
- DOI: 10.1002/jcph.2139
Extrapolation of Adult Efficacy Data to Pediatric Systemic Lupus Erythematosus: Evaluating Similarities in Exposure-Response
Abstract
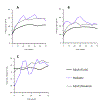
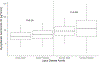
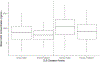
To streamline drug development, the United States Food and Drug Administration (FDA) can consider the extrapolation of adult efficacy data to children when the disease and drug effects are sufficiently similar. This study explored whether the relationship between drug exposure and response for selected drugs in systemic lupus erythematosus (SLE) was sufficiently similar to support a consideration of the extrapolation of adult efficacy data to children of ≥5 years of age. An exposure-response analysis of drugs used to treat SLE was conducted using published exposure versus response and efficacy versus time data. Statistical analyses included noncompartmental analysis of a drug's area under the effect curve and direct Imax pharmacodynamic (PD) modeling. Six drugs were included: azathioprine, belimumab, cyclophosphamide, hydroxychloroquine, mycophenolate/mycophenolic acid, and rituximab. For belimumab, the net change in responders at week 52 (the primary end point) was nearly identical between 1 adult trial and the pediatric trial. For mycophenolate, PD modeling suggested no significant differences in exposure and SLE disease activity between adults and children. For azathioprine, cyclophosphamide, hydroxychloroquine, and rituximab the data were not sufficient to quantitatively characterize the exposure-response relationship, but the clinical or pharmacologic response between children and adults was similar overall. Adult SLE data should be leveraged to guide pediatric drug development programs and identify areas with residual uncertainty regarding the effectiveness or safety of a drug in children. The degree to which efficacy extrapolation can reduce clinical trial requirements in pediatric SLE should be individualized for each new drug product, depending in part on the mechanism of action of the drug and the similarity of disease manifestations in children and adults.
Keywords: pediatrics; pharmacodynamics; pharmacokinetics and drug metabolism; regulatory/scientific affairs; rheumatology.
© 2022, The American College of Clinical Pharmacology.
Conflict of interest statement
Figures

References
-
- Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128(5):e1242–1249. - PubMed
-
- Product Label. Belimumab injection. GlaxoSmithKline LP, PA. March 11, 2021.
-
- Livingston B, Bonner A, Pope J. Differences in clinical manifestations between childhood-onset lupus and adult-onset lupus: a meta-analysis. Lupus. 2011;20(13):1345–1355. - PubMed
-
- United States Food and Drug Administration (FDA). Pediatric science and research activities. FDA web site. https://www.fda.gov/science-research/pediatrics/pediatric-science-and-re.... Updated October 15, 2021. Accessed October 25, 2021.
-
- United States Food and Drug Administration (FDA). Guidance document: general clinical pharmacology considerations for pediatric studies for drugs and biological products. December 2014. FDA web site. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat.... Accessed October 25, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

