Expected Impact of Universal Immunization With Nirsevimab Against RSV-Related Outcomes and Costs Among All US Infants in Their First RSV Season: A Static Model
- PMID: 35968866
- PMCID: PMC9377043
- DOI: 10.1093/infdis/jiac216
Expected Impact of Universal Immunization With Nirsevimab Against RSV-Related Outcomes and Costs Among All US Infants in Their First RSV Season: A Static Model
Abstract
Background: Respiratory syncytial virus (RSV) is associated with substantial morbidity in the United States, especially among infants. Nirsevimab, an investigational long-acting monoclonal antibody, was evaluated as an immunoprophylactic strategy for infants in their first RSV season and for its potential impact on RSV-associated, medically attended lower respiratory tract illness (RSV-MALRTI) and associated costs.
Methods: A static decision-analytic model of the US birth cohort during its first RSV season was developed to estimate nirsevimab's impact on RSV-related health events and costs; model inputs included US-specific costs and epidemiological data. Modelled RSV-related outcomes included primary care and emergency room visits, hospitalizations including intensive care unit admission and mechanical ventilations, and RSV-related mortality.
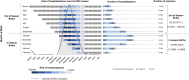
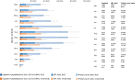
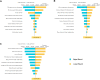
Results: Under current standard of care, RSV caused 529 915 RSV-MALRTIs and 47 281 hospitalizations annually, representing $1.2 billion (2021 US dollars [USD]) in costs. Universal immunization of all infants with nirsevimab is expected to reduce 290 174 RSV-MALRTI, 24 986 hospitalizations, and expenditures of $612 million 2021 USD.
Conclusions: An all-infant immunization strategy with nirsevimab could substantially reduce the health and economic burden for US infants during their first RSV season. While this reduction is driven by term infants, all infants, including palivizumab-eligible and preterm infants, would benefit from this strategy.
Keywords: RSV; United States; burden; cost; economics; infants; lower respiratory tract illness; model; nirsevimab.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. K., M. B., and J. L. are employees of Sanofi and may hold shares and/or stock options in the company. A. S., R. M., S. M., and J. R. are salaried employees of Evidera and are not allowed to accept remuneration from any clients for their services. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
Accurately Assessing the Expected Impact of Universal First Respiratory Syncytial Virus (RSV) Season Immunization With Nirsevimab Against RSV-Related Outcomes and Costs Among All US Infants.J Infect Dis. 2023 May 29;227(11):1333-1334. doi: 10.1093/infdis/jiac488. J Infect Dis. 2023. PMID: 36520978 No abstract available.
References
-
- Shi N, Palmer L, Chu BC, et al. . Association of RSV lower respiratory tract infection and subsequent healthcare use and costs: a Medicaid claims analysis in early-preterm, late-preterm, and full-term infants. J Med Econ 2011; 14:335–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

