RBD-VLP Vaccines Adjuvanted with Alum or SWE Protect K18-hACE2 Mice against SARS-CoV-2 VOC Challenge
- PMID: 35968964
- PMCID: PMC9429941
- DOI: 10.1128/msphere.00243-22
RBD-VLP Vaccines Adjuvanted with Alum or SWE Protect K18-hACE2 Mice against SARS-CoV-2 VOC Challenge
Abstract
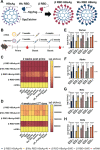
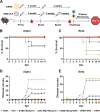
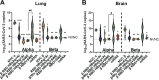
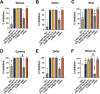
The ongoing COVID-19 pandemic has contributed largely to the global vaccine disparity. Development of protein subunit vaccines can help alleviate shortages of COVID-19 vaccines delivered to low-income countries. Here, we evaluated the efficacy of a three-dose virus-like particle (VLP) vaccine composed of hepatitis B surface antigen (HBsAg) decorated with the receptor binding domain (RBD) from the Wuhan or Beta SARS-CoV-2 strain adjuvanted with either aluminum hydroxide (alum) or squalene in water emulsion (SWE). RBD HBsAg vaccines were compared to the standard two doses of Pfizer mRNA vaccine. Alum-adjuvanted vaccines were composed of either HBsAg conjugated with Beta RBD alone (β RBD HBsAg+Al) or a combination of both Beta RBD HBsAg and Wuhan RBD HBsAg (β/Wu RBD HBsAg+Al). RBD vaccines adjuvanted with SWE were formulated with Beta RBD HBsAg (β RBD HBsAg+SWE) or without HBsAg (β RBD+SWE). Both alum-adjuvanted RBD HBsAg vaccines generated functional RBD IgG against multiple SARS-CoV-2 variants of concern (VOC), decreased viral RNA burden, and lowered inflammation in the lung against Alpha or Beta challenge in K18-hACE2 mice. However, only β/Wu RBD HBsAg+Al was able to afford 100% survival to mice challenged with Alpha or Beta VOC. Furthermore, mice immunized with β RBD HBsAg+SWE induced cross-reactive neutralizing antibodies against major VOC of SARS-CoV-2, lowered viral RNA burden in the lung and brain, and protected mice from Alpha or Beta challenge similarly to mice immunized with Pfizer mRNA. However, RBD+SWE immunization failed to protect mice from VOC challenge. Our findings demonstrate that RBD HBsAg VLP vaccines provided similar protection profiles to the approved Pfizer mRNA vaccines used worldwide and may offer protection against SARS-CoV-2 VOC. IMPORTANCE Global COVID-19 vaccine distribution to low-income countries has been a major challenge of the pandemic. To address supply chain issues, RBD virus-like particle (VLP) vaccines that are cost-effective and capable of large-scale production were developed and evaluated for efficacy in preclinical mouse studies. We demonstrated that RBD-VLP vaccines protected K18-hACE2 mice against Alpha or Beta challenge similarly to Pfizer mRNA vaccination. Our findings showed that the VLP platform can be utilized to formulate immunogenic and efficacious COVID-19 vaccines.
Keywords: COVID-19; HBsAg; RBD; SARS-CoV-2; SWE; SpyCatcher; SpyTag; VLP; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Intranasal VLP-RBD vaccine adjuvanted with BECC470 confers immunity against Delta SARS-CoV-2 challenge in K18-hACE2-mice.Vaccine. 2023 Jul 31;41(34):5003-5017. doi: 10.1016/j.vaccine.2023.06.080. Epub 2023 Jun 28. Vaccine. 2023. PMID: 37407405 Free PMC article.
-
Evaluating Antibody Mediated Protection against Alpha, Beta, and Delta SARS-CoV-2 Variants of Concern in K18-hACE2 Transgenic Mice.J Virol. 2022 Mar 23;96(6):e0218421. doi: 10.1128/jvi.02184-21. Epub 2022 Jan 26. J Virol. 2022. PMID: 35080423 Free PMC article.
-
Passive immunization with equine RBD-specific Fab protects K18-hACE2-mice against Alpha or Beta variants of SARS-CoV-2.Front Immunol. 2022 Aug 15;13:948431. doi: 10.3389/fimmu.2022.948431. eCollection 2022. Front Immunol. 2022. PMID: 36091051 Free PMC article.
-
Nanoparticle and virus-like particle vaccine approaches against SARS-CoV-2.J Microbiol. 2022 Mar;60(3):335-346. doi: 10.1007/s12275-022-1608-z. Epub 2022 Jan 28. J Microbiol. 2022. PMID: 35089583 Free PMC article. Review.
-
COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions.Front Cell Infect Microbiol. 2021 Sep 10;11:690621. doi: 10.3389/fcimb.2021.690621. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34568087 Free PMC article. Review.
Cited by
-
Chimeric Hepatitis B core virus-like particles harboring SARS-CoV2 epitope elicit a humoral immune response in mice.Microb Cell Fact. 2023 Feb 25;22(1):39. doi: 10.1186/s12934-023-02043-z. Microb Cell Fact. 2023. PMID: 36841778 Free PMC article.
-
Respiratory Viruses and Virus-like Particle Vaccine Development: How Far Have We Advanced?Viruses. 2023 Jan 30;15(2):392. doi: 10.3390/v15020392. Viruses. 2023. PMID: 36851606 Free PMC article. Review.
-
Intranasal VLP-RBD vaccine adjuvanted with BECC470 confers immunity against Delta SARS-CoV-2 challenge in K18-hACE2-mice.Vaccine. 2023 Jul 31;41(34):5003-5017. doi: 10.1016/j.vaccine.2023.06.080. Epub 2023 Jun 28. Vaccine. 2023. PMID: 37407405 Free PMC article.
-
Exigency of Plant-Based Vaccine against COVID-19 Emergence as Pandemic Preparedness.Vaccines (Basel). 2023 Aug 9;11(8):1347. doi: 10.3390/vaccines11081347. Vaccines (Basel). 2023. PMID: 37631915 Free PMC article. Review.
-
Self-assembling SARS-CoV-2 spike-HBsAg nanoparticles elicit potent and durable neutralizing antibody responses via genetic delivery.NPJ Vaccines. 2023 Aug 8;8(1):111. doi: 10.1038/s41541-023-00707-w. NPJ Vaccines. 2023. PMID: 37553406 Free PMC article.
References
-
- Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, Schmitz KS, Rijsbergen LC, van Osch JAT, Dijkhuizen E, Smits G, Comvalius A, van Mourik D, Caniels TG, van Gils MJ, Sanders RW, Oude Munnink BB, Molenkamp R, de Jager HJ, Haagmans BL, de Swart RL, Koopmans MPG, van Binnendijk RS, de Vries RD, GeurtsvanKessel CH. 2021. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci Immunol 6:eabj1750. doi:10.1126/sciimmunol.abj1750. - DOI - PMC - PubMed
-
- Aleem A, Samad Akbar Bari A, Slenker KA. 2022. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). StatPearls Publishing, Treasure Island, FL. - PubMed
-
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Péré H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Lorière E, Rey FA, Schwartz O. 2021. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596:276–280. doi:10.1038/s41586-021-03777-9. - DOI - PubMed
-
- Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, Datir R, Collier DA, Albecka A, Singh S, Pandey R, Brown J, Zhou J, Goonawardane N, Mishra S, Whittaker C, Mellan T, Marwal R, Datta M, Sengupta S, Ponnusamy K, Radhakrishnan VS, Abdullahi A, Charles O, Chattopadhyay P, Devi P, Caputo D, Peacock T, Wattal C, Goel N, Satwik A, Vaishya R, Agarwal M, Chauhan H, Dikid T, Gogia H, Lall H, Verma K, Dhar MS, Singh MK, Soni N, Meena N, Madan P, Singh P, Sharma R, Sharma R, Kabra S, Kumar S, Kumari S, Sharma U, The Indian SARS-CoV-2 Genomics Consortium (INSACOG), et al. . 2021. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 599:114–119. doi:10.1038/s41586-021-03944-y. - DOI - PMC - PubMed
-
- Liu L, Iketani S, Guo Y, Chan JF-W, Wang M, Liu L, Luo Y, Chu H, Huang Y, Nair MS, Yu J, Chik KK-H, Yuen TT-T, Yoon C, To KK-W, Chen H, Yin MT, Sobieszczyk ME, Huang Y, Wang HH, Sheng Z, Yuen K-Y, Ho DD. 2022. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 602:676–681. doi:10.1038/s41586-021-04388-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

