PLK4 drives centriole amplification and apical surface area expansion in multiciliated cells
- PMID: 35969030
- PMCID: PMC9507127
- DOI: 10.7554/eLife.80643
PLK4 drives centriole amplification and apical surface area expansion in multiciliated cells
Abstract
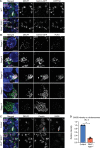
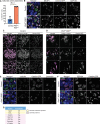
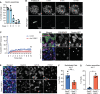
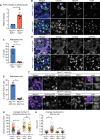
Multiciliated cells (MCCs) are terminally differentiated epithelia that assemble multiple motile cilia used to promote fluid flow. To template these cilia, MCCs dramatically expand their centriole content during a process known as centriole amplification. In cycling cells, the master regulator of centriole assembly Polo-like kinase 4 (PLK4) is essential for centriole duplication; however recent work has questioned the role of PLK4 in centriole assembly in MCCs. To address this discrepancy, we created genetically engineered mouse models and demonstrated that both PLK4 protein and kinase activity are critical for centriole amplification in MCCs. Tracheal epithelial cells that fail centriole amplification accumulate large assemblies of centriole proteins and do not undergo apical surface area expansion. These results show that the initial stages of centriole assembly are conserved between cycling cells and MCCs and suggest that centriole amplification and surface area expansion are coordinated events.
Keywords: Plk4; cell biology; centriole; cilia; deuterosome; mouse; multiciliated.
Plain language summary
Every day, we inhale thousands of viruses, bacteria and pollution particles. To protect against these threats, cells in our airways produce mucus that traps inhaled particles before they reach the lungs. This mucus then needs to be removed to prevent it from becoming a breeding ground for microbes that may cause a respiratory infection. This is the responsibility of cells covered in tiny hair-like structures called cilia that move together to propel the mucus-trapped particles out of the airways. These specialized cells can have up to 300 motile cilia on their surface, which grow from structures called centrioles that then anchor the cilia in place. Multiciliated cells are generated from precursor cells that only have two centrioles. Therefore, as these precursors develop, they must produce large numbers of centrioles, considerably more than other cells that only need a couple of extra centrioles during cell division. However, recent studies have questioned whether the precursors of multiciliated cells rely on the same regulatory proteins to produce centrioles as dividing cells. To help answer this question, LoMastro et al. created genetically engineered mice that lacked or had an inactive form of PLK4, a protein which controls centriole formation in all cell types lacking multiple cilia. This showed that multiciliated cells also need this protein to produce centrioles. LoMastro et al. also found that multiciliated cells became larger while building centrioles, suggesting that this amplification process helps control the cell’s final size. Defects in motile cilia activity can lead to fluid build-up in the brain, respiratory infections and infertility. Unfortunately, these disorders are difficult to diagnose currently and there is no cure. The findings of LoMastro et al. further our understanding of how motile cilia are built and maintained, and may help future scientists to develop better diagnostic tools and treatments for patients.
© 2022, LoMastro et al.
Conflict of interest statement
GL, CD, AM, CJ, MS, AH No competing interests declared
Figures
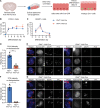
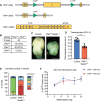


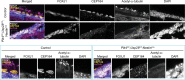
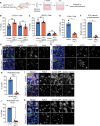
Comment in
-
One master to rule them all.Elife. 2022 Sep 23;11:e82873. doi: 10.7554/eLife.82873. Elife. 2022. PMID: 36149392 Free PMC article.
Similar articles
-
Regulation of cilia abundance in multiciliated cells.Elife. 2019 Apr 26;8:e44039. doi: 10.7554/eLife.44039. Elife. 2019. PMID: 31025935 Free PMC article.
-
Dynamics of centriole amplification in centrosome-depleted brain multiciliated progenitors.Sci Rep. 2019 Sep 10;9(1):13060. doi: 10.1038/s41598-019-49416-2. Sci Rep. 2019. PMID: 31506528 Free PMC article.
-
Deuterosome-mediated centriole biogenesis.Dev Cell. 2013 Oct 14;27(1):103-12. doi: 10.1016/j.devcel.2013.08.021. Epub 2013 Sep 26. Dev Cell. 2013. PMID: 24075808 Free PMC article.
-
Emerging Picture of Deuterosome-Dependent Centriole Amplification in MCCs.Cells. 2018 Sep 27;7(10):152. doi: 10.3390/cells7100152. Cells. 2018. PMID: 30262752 Free PMC article. Review.
-
Controlling centriole numbers: Geminin family members as master regulators of centriole amplification and multiciliogenesis.Chromosoma. 2018 Jun;127(2):151-174. doi: 10.1007/s00412-017-0652-7. Epub 2017 Dec 14. Chromosoma. 2018. PMID: 29243212 Review.
Cited by
-
Centrioles are frequently amplified in early B cell development but dispensable for humoral immunity.Nat Commun. 2024 Oct 15;15(1):8890. doi: 10.1038/s41467-024-53222-4. Nat Commun. 2024. PMID: 39406735 Free PMC article.
-
One master to rule them all.Elife. 2022 Sep 23;11:e82873. doi: 10.7554/eLife.82873. Elife. 2022. PMID: 36149392 Free PMC article.
-
PLK4 self-phosphorylation drives the selection of a single site for procentriole assembly.J Cell Biol. 2023 Dec 4;222(12):e202301069. doi: 10.1083/jcb.202301069. Epub 2023 Sep 29. J Cell Biol. 2023. PMID: 37773039 Free PMC article.
-
Rapid and sustained degradation of the essential centrosome protein CEP192 in live mice using the AID2 system.Sci Adv. 2025 Feb 28;11(9):eadq2339. doi: 10.1126/sciadv.adq2339. Epub 2025 Feb 28. Sci Adv. 2025. PMID: 40020058 Free PMC article.
-
The DNA damage response pathway is required for multiciliated cell differentiation.bioRxiv [Preprint]. 2025 Jul 14:2025.07.13.664567. doi: 10.1101/2025.07.13.664567. bioRxiv. 2025. PMID: 40791460 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

