Structures of topoisomerase V in complex with DNA reveal unusual DNA-binding mode and novel relaxation mechanism
- PMID: 35969036
- PMCID: PMC9489208
- DOI: 10.7554/eLife.72702
Structures of topoisomerase V in complex with DNA reveal unusual DNA-binding mode and novel relaxation mechanism
Abstract
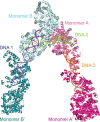
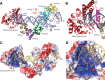
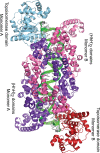
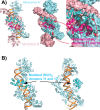
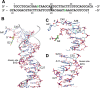
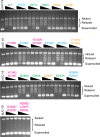
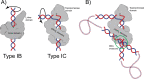
Topoisomerase V is a unique topoisomerase that combines DNA repair and topoisomerase activities. The enzyme has an unusual arrangement, with a small topoisomerase domain followed by 12 tandem (HhH)2 domains, which include 3 AP lyase repair domains. The uncommon architecture of this enzyme bears no resemblance to any other known topoisomerase. Here, we present structures of topoisomerase V in complex with DNA. The structures show that the (HhH)2 domains wrap around the DNA and in this manner appear to act as a processivity factor. There is a conformational change in the protein to expose the topoisomerase active site. The DNA bends sharply to enter the active site, which melts the DNA and probably facilitates relaxation. The structures show a DNA-binding mode not observed before and provide information on the way this atypical topoisomerase relaxes DNA. In common with type IB enzymes, topoisomerase V relaxes DNA using a controlled rotation mechanism, but the structures show that topoisomerase V accomplishes this in different manner. Overall, the structures firmly establish that type IC topoisomerases form a distinct type of topoisomerases, with no similarities to other types at the sequence, structural, or mechanistic level. They represent a completely different solution to DNA relaxation.
Keywords: Methanopyrus kandleri; archaea; methanogen; molecular biophysics; structural biology.
© 2022, Osterman and Mondragón.
Conflict of interest statement
AO, AM No competing interests declared
Figures







References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallographica. Section D, Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Ahmad M, Xue Y, Lee SK, Martindale JL, Shen W, Li W, Zou S, Ciaramella M, Debat H, Nadal M, Leng F, Zhang H, Wang Q, Siaw GE-L, Niu H, Pommier Y, Gorospe M, Hsieh T-S, Tse-Dinh Y-C, Xu D, Wang W. RNA topoisomerase is prevalent in all domains of life and associates with polyribosomes in animals. Nucleic Acids Research. 2016;44:6335–6349. doi: 10.1093/nar/gkw508. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

