IFI16 Partners with KAP1 to Maintain Epstein-Barr Virus Latency
- PMID: 35969079
- PMCID: PMC9472614
- DOI: 10.1128/jvi.01028-22
IFI16 Partners with KAP1 to Maintain Epstein-Barr Virus Latency
Abstract
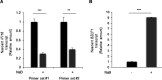
Herpesviruses establish latency to ensure permanent residence in their hosts. Upon entry into a cell, these viruses are rapidly silenced by the host, thereby limiting the destructive viral lytic phase while allowing the virus to hide from the immune system. Notably, although the establishment of latency by the oncogenic herpesvirus Epstein-Barr virus (EBV) requires the expression of viral latency genes, latency can be maintained with a negligible expression of viral genes. Indeed, in several herpesviruses, the host DNA sensor IFI16 facilitated latency via H3K9me3 heterochromatinization. This silencing mark is typically imposed by the constitutive heterochromatin machinery (HCM). The HCM, in an antiviral role, also silences the lytic phase of EBV and other herpes viruses. We investigated if IFI16 restricted EBV lytic activation by partnering with the HCM and found that IFI16 interacted with core components of the HCM, including the KRAB-associated protein 1 (KAP1) and the site-specific DNA binding KRAB-ZFP SZF1. This partnership silenced the EBV lytic switch protein ZEBRA, encoded by the BZLF1 gene, thereby favoring viral latency. Indeed, IFI16 contributed to H3K9 trimethylation at lytic genes of all kinetic classes. In defining topology, we found that IFI16 coenriched with KAP1 at the BZLF1 promoter, and while IFI16 and SZF1 were each adjacent to KAP1 in latent cells, IFI16 and SZF1 were not. Importantly, we also found that disruption of latency involved rapid downregulation of IFI16 transcription. These findings revealed a previously unknown partnership between IFI16 and the core HCM that supports EBV latency via antiviral heterochromatic silencing. IMPORTANCE The interferon-gamma inducible protein 16 (IFI16) is a nuclear DNA sensor that mediates antiviral responses by activating the inflammasome, triggering an interferon response, and silencing lytic genes of herpesviruses. The last, which helps maintain latency of the oncoherpesvirus Epstein-Barr virus (EBV), is accomplished via H3K9me3 heterochromatinization through unknown mechanisms. Here, we report that IFI16 physically partners with the core constitutive heterochromatin machinery to silence the key EBV lytic switch protein, thereby ensuring continued viral latency in B lymphocytes. We also find that disruption of latency involves rapid transcriptional downregulation of IFI16. These findings point to hitherto unknown physical and functional partnerships between a well-known antiviral mechanism and the core components of the constitutive heterochromatin machinery.
Keywords: Epstein-Barr virus; H3K9me3; IFI16; KAP1; TRIM28; ZEBRA; ZTA; herpesvirus; heterochromatin machinery; latency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lo Cigno I, De Andrea M, Borgogna C, Albertini S, Landini MM, Peretti A, Johnson KE, Chandran B, Landolfo S, Gariglio M. 2015. The nuclear DNA sensor IFI16 acts as a restriction factor for human papillomavirus replication through epigenetic modifications of the viral promoters. J Virol 89:7506–7520. 10.1128/JVI.00013-15. - DOI - PMC - PubMed
-
- Johnson KE, Bottero V, Flaherty S, Dutta S, Singh VV, Chandran B. 2014. IFI16 restricts HSV-1 replication by accumulating on the hsv-1 genome, repressing HSV-1 gene expression, and directly or indirectly modulating histone modifications. PLoS Pathog 10:e1004503. 10.1371/journal.ppat.1004503. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

