Herpes Simplex Virus 1 Can Bypass Impaired Epidermal Barriers upon Ex Vivo Infection of Skin from Atopic Dermatitis Patients
- PMID: 35969080
- PMCID: PMC9472615
- DOI: 10.1128/jvi.00864-22
Herpes Simplex Virus 1 Can Bypass Impaired Epidermal Barriers upon Ex Vivo Infection of Skin from Atopic Dermatitis Patients
Abstract
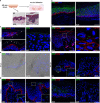
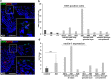
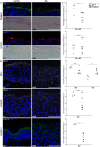
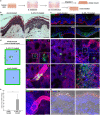
To infect its human host, herpes simplex virus 1 (HSV-1) must overcome the protective barriers of skin and mucosa. Here, we addressed whether pathological skin conditions can facilitate viral entry via the skin surface and used ex vivo infection studies to explore viral invasion in atopic dermatitis (AD) skin characterized by disturbed barrier functions. Our focus was on the visualization of the onset of infection in single cells to determine the primary entry portals in the epidermis. After ex vivo infection of lesional AD skin, we observed infected cells in suprabasal layers indicating successful invasion in the epidermis via the skin surface which was never detected in control skin where only sample edges allowed viral access. The redistribution of filaggrin, loricrin, and tight-junction components in the lesional skin samples suggested multiple defective mechanical barriers. To dissect the parameters that contribute to HSV-1 invasion, we induced an AD-like phenotype by adding the Th2 cytokines interleukin 4 (IL-4) and IL-13 to healthy human skin samples. Strikingly, we detected infected cells in the epidermis, implying that the IL-4/IL-13-driven inflammation is sufficient to induce modifications allowing HSV-1 to penetrate the skin surface. In summary, not only did lesional AD skin facilitate HSV-1 penetration but IL-4/IL-13 responses alone allowed virus invasion. Our results suggest that the defective epidermal barriers of AD skin and the inflammation-induced altered barriers in healthy skin can make receptors accessible for HSV-1. IMPORTANCE Herpes simplex virus 1 (HSV-1) can target skin to establish primary infection in the epithelium. While the human skin provides effective barriers against viral invasion under healthy conditions, a prominent example of successful invasion is the disseminated HSV-1 infection in the skin of atopic dermatitis (AD) patients. AD is characterized by impaired epidermal barrier functions, chronic inflammation, and dysbiosis of skin microbiota. We addressed the initial invasion process of HSV-1 in atopic dermatitis skin to understand whether the physical barrier functions are sufficiently disturbed to allow the virus to invade skin and reach its receptors on skin cells. Our results demonstrate that HSV-1 can indeed penetrate and initiate infection in atopic dermatitis skin. Since treatment of skin with IL-4 and IL-13 already resulted in successful invasion, we assume that inflammation-induced barrier defects play an important role for the facilitated access of HSV-1 to its target cells.
Keywords: HSV-1; IL-4/IL-13; atopic dermatitis; epidermal barriers; human skin; viral entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ex Vivo Infection of Human Skin Models with Herpes Simplex Virus 1: Accessibility of the Receptor Nectin-1 during Formation or Impairment of Epidermal Barriers Is Restricted by Tight Junctions.J Virol. 2023 Jun 29;97(6):e0026223. doi: 10.1128/jvi.00262-23. Epub 2023 Jun 8. J Virol. 2023. PMID: 37289055 Free PMC article.
-
Ex Vivo Infection of Human Skin with Herpes Simplex Virus 1 Reveals Mechanical Wounds as Insufficient Entry Portals via the Skin Surface.J Virol. 2021 Oct 13;95(21):e0133821. doi: 10.1128/JVI.01338-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379501 Free PMC article.
-
Mechanical Barriers Restrict Invasion of Herpes Simplex Virus 1 into Human Oral Mucosa.J Virol. 2017 Oct 27;91(22):e01295-17. doi: 10.1128/JVI.01295-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878080 Free PMC article.
-
Eczema Herpeticum: Clinical and Pathophysiological Aspects.Clin Rev Allergy Immunol. 2020 Aug;59(1):1-18. doi: 10.1007/s12016-019-08768-3. Clin Rev Allergy Immunol. 2020. PMID: 31836943 Review.
-
Current aspects of innate and adaptive immunity in atopic dermatitis.Clin Rev Allergy Immunol. 2007 Oct;33(1-2):35-44. doi: 10.1007/s12016-007-0032-9. Clin Rev Allergy Immunol. 2007. PMID: 18094945 Review.
Cited by
-
Impaired endocytosis and accumulation in early endosomal compartments defines herpes simplex virus-mediated disruption of the nonclassical MHC class I-related molecule MR1.J Biol Chem. 2024 Oct;300(10):107748. doi: 10.1016/j.jbc.2024.107748. Epub 2024 Sep 12. J Biol Chem. 2024. PMID: 39260697 Free PMC article.
-
Regulation of inflammatory cytokines and activation of PI3K/Akt pathway by Yiqi Jiedu Formula in recurrent Herpes Simplex Keratitis: Experimental and network pharmacology evidence.Virus Res. 2025 May;355:199561. doi: 10.1016/j.virusres.2025.199561. Epub 2025 Mar 20. Virus Res. 2025. PMID: 40120648 Free PMC article.
-
An Intrinsic Host Defense against HSV-1 Relies on the Activation of Xenophagy with the Active Clearance of Autophagic Receptors.Cells. 2024 Jul 26;13(15):1256. doi: 10.3390/cells13151256. Cells. 2024. PMID: 39120287 Free PMC article.
-
The Expression of Plasmacytoid Dendritic Cells and TLR7/9-MyD88-IRAKs Pathway in Chronic Eczema Lesions.Clin Cosmet Investig Dermatol. 2023 Apr 24;16:1079-1087. doi: 10.2147/CCID.S405491. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 37123625 Free PMC article.
-
Breaching the Barrier: Investigating Initial Herpes Simplex Viral Infection and Spread in Human Skin and Mucosa.Viruses. 2024 Nov 18;16(11):1790. doi: 10.3390/v16111790. Viruses. 2024. PMID: 39599904 Free PMC article. Review.
References
-
- Singh M, Goodyear HM, Breuer J. 2020. Herpes simplex virus infections. In Hoeger P, Kinsler V, Yan A, Harper J, Oranje A, Bodemer C, Larralde M, Luk D, Mendiratta V, Purvis D (ed), Harper’s textbook of pediatric dermatology, 4th ed. Wiley, New York, NY.
-
- Goodyear HM. 2011. Eczema herpeticum. In Irvine AD, Hoeger PH, Yan AC (ed), Harper’s textbook of pediatric dermatology, 3rd ed. Wiley, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

