Measuring Skeletal Muscle Thermogenesis in Mice and Rats
- PMID: 35969093
- PMCID: PMC9969793
- DOI: 10.3791/64264
Measuring Skeletal Muscle Thermogenesis in Mice and Rats
Abstract
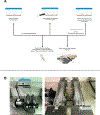
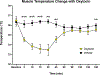
Skeletal muscle thermogenesis provides a potential avenue for better understanding metabolic homeostasis and the mechanisms underlying energy expenditure. Surprisingly little evidence is available to link the neural, myocellular, and molecular mechanisms of thermogenesis directly to measurable changes in muscle temperature. This paper describes a method in which temperature transponders are utilized to retrieve direct measurements of mouse and rat skeletal muscle temperature. Remote transponders are surgically implanted within the muscle of mice and rats, and the animals are given time to recover. Mice and rats must then be repeatedly habituated to the testing environment and procedure. Changes in muscle temperature are measured in response to pharmacological or contextual stimuli in the home cage. Muscle temperature can also be measured during prescribed physical activity (i.e., treadmill walking at a constant speed) to factor out changes in activity as contributors to the changes in muscle temperature induced by these stimuli. This method has been successfully used to elucidate mechanisms underlying muscle thermogenic control at the level of the brain, sympathetic nervous system, and skeletal muscle. Provided are demonstrations of this success using predator odor (PO; ferret odor) as a contextual stimulus and injections of oxytocin (Oxt) as a pharmacological stimulus, where predator odor induces muscle thermogenesis, and Oxt suppresses muscle temperature. Thus, these datasets display the efficacy of this method in detecting rapid changes in muscle temperature.
Conflict of interest statement
Disclosures
The authors declare that they have no conflicts of interest.
Figures

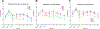

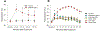
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
