Step-by-step diagnosis and management of the nocebo/drucebo effect in statin-associated muscle symptoms patients: a position paper from the International Lipid Expert Panel (ILEP)
- PMID: 35969116
- PMCID: PMC9178378
- DOI: 10.1002/jcsm.12960
Step-by-step diagnosis and management of the nocebo/drucebo effect in statin-associated muscle symptoms patients: a position paper from the International Lipid Expert Panel (ILEP)
Abstract
Statin intolerance is a clinical syndrome whereby adverse effects (AEs) associated with statin therapy [most commonly statin-associated muscle symptoms (SAMS)] result in the discontinuation of therapy and consequently increase the risk of adverse cardiovascular outcomes. However, complete statin intolerance occurs in only a small minority of treated patients (estimated prevalence of only 3-5%). Many perceived AEs are misattributed (e.g. physical musculoskeletal injury and inflammatory myopathies), and subjective symptoms occur as a result of the fact that patients expect them to do so when taking medicines (the nocebo/drucebo effect)-what might be truth even for over 50% of all patients with muscle weakness/pain. Clear guidance is necessary to enable the optimal management of plasma in real-world clinical practice in patients who experience subjective AEs. In this Position Paper of the International Lipid Expert Panel (ILEP), we present a step-by-step patient-centred approach to the identification and management of SAMS with a particular focus on strategies to prevent and manage the nocebo/drucebo effect and to improve long-term compliance with lipid-lowering therapy.
Keywords: Drucebo effect; Nocebo effect; SAMS; Statin intolerance.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Figures
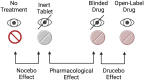
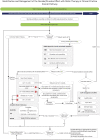

References
-
- Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein‐cholesterol and cardiovascular events with proprotein convertase subtilisin‐kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J 2018;39:2540–2545. - PMC - PubMed
-
- Reiner Z. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol 2013;10:453–464. - PubMed
-
- Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. - PubMed
-
- Thompson PD, Panza G, Zaleski A, Taylor B. Statin‐sssociated side effects. J Am Coll Cardiol 2016;67:2395–2410. - PubMed
-
- Simic I, Reiner Z. Adverse effects of statins—myths and reality. Curr Pharm Des 2015;21:1220–1226. - PubMed

