ZOOMICS : comparative metabolomics of red blood cells from dogs, cows, horses and donkeys during refrigerated storage for up to 42 days
- PMID: 35969134
- PMCID: PMC10335344
- DOI: 10.2450/2022.0118-22
ZOOMICS : comparative metabolomics of red blood cells from dogs, cows, horses and donkeys during refrigerated storage for up to 42 days
Abstract
Background: The use of omics technologies in human transfusion medicine has improved our understanding of the red blood cell (RBC) storage lesion(s). Despite significant progress towards understanding the storage lesion(s) of human RBCs, a comparison of basal and post-storage RBC metabolism across multiple species using omics technologies has not yet been reported, and is the focus of this study.
Materials and methods: Blood was collected in a standard bag system (CPD-SAG-Mannitol) from dogs (n=8), horses, bovines, and donkeys (n=6). All bags were stored at 4°C for up to 42 days (i.e., the end of the shelf life in Italian veterinary clinics) and sampled weekly for metabolomics analyses. In addition, data comparisons to our ongoing Zoomics project are included to compare this study's results with those of non-human primates and humans.
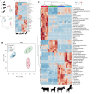
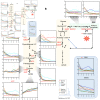
Results: Significant interspecies differences in RBC metabolism were observed at baseline, at the time of donation, with bovine showing significantly higher levels of metabolites in the tryptophan/kynurenine pathway; dogs showing elevated levels of high-energy compounds (especially adenosine triphosphate and S-adenosyl-methionine) and equine (donkey and horse) RBCs showing almost overlapping phenotypes, with the highest levels of free branched chain amino acids, glycolytic metabolites (including 2,3-diphosphoglycerate), higher total glutathione pools, and elevated metabolites of the folate pathway compared to the other species. Strikingly, previously described metabolic markers of the storage lesion(s) in humans followed similar trends across all species, though the rate of accumulation/depletion of metabolites in energy and redox metabolism varied by species, with equine blood showing the lowest degree of storage lesion(s).
Discussion: These results interrogate RBC metabolism across a range of mammalian species and improve our understanding of both human and veterinary blood storage and transfusion.
Conflict of interest statement
Though unrelated to the contents of this manuscripts, the authors declare that AD and TN are founders of Omix Technologies Inc. AD is also a consultant for Altis Biosciences LLC., Rubius Inc. and Forma Inc. AD and SLS are both consultants for Hemanext Inc. SLS is also a consultant for Tioma, Inc., TCIP, Inc., and the Executive Director of the Worldwide Initiative for Rh Disease Eradication (WIRhE). All the other authors disclose no conflicts of interest relevant to this study.
Figures




References
-
- D’Alessandro A, Culp-Hill R, Reisz JA, Anderson M, Fu X, Nemkov T, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59:89–100. doi: 10.1111/trf.14979. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
