Syndrome of Combined Pulmonary Fibrosis and Emphysema: An Official ATS/ERS/JRS/ALAT Research Statement
- PMID: 35969190
- PMCID: PMC7615200
- DOI: 10.1164/rccm.202206-1041ST
Syndrome of Combined Pulmonary Fibrosis and Emphysema: An Official ATS/ERS/JRS/ALAT Research Statement
Abstract
Background: The presence of emphysema is relatively common in patients with fibrotic interstitial lung disease. This has been designated combined pulmonary fibrosis and emphysema (CPFE). The lack of consensus over definitions and diagnostic criteria has limited CPFE research. Goals: The objectives of this task force were to review the terminology, definition, characteristics, pathophysiology, and research priorities of CPFE and to explore whether CPFE is a syndrome. Methods: This research statement was developed by a committee including 19 pulmonologists, 5 radiologists, 3 pathologists, 2 methodologists, and 2 patient representatives. The final document was supported by a focused systematic review that identified and summarized all recent publications related to CPFE. Results: This task force identified that patients with CPFE are predominantly male, with a history of smoking, severe dyspnea, relatively preserved airflow rates and lung volumes on spirometry, severely impaired DlCO, exertional hypoxemia, frequent pulmonary hypertension, and a dismal prognosis. The committee proposes to identify CPFE as a syndrome, given the clustering of pulmonary fibrosis and emphysema, shared pathogenetic pathways, unique considerations related to disease progression, increased risk of complications (pulmonary hypertension, lung cancer, and/or mortality), and implications for clinical trial design. There are varying features of interstitial lung disease and emphysema in CPFE. The committee offers a research definition and classification criteria and proposes that studies on CPFE include a comprehensive description of radiologic and, when available, pathological patterns, including some recently described patterns such as smoking-related interstitial fibrosis. Conclusions: This statement delineates the syndrome of CPFE and highlights research priorities.
Keywords: diagnosis; emphysema; fibrosis; interstitial lung disease; management.
Figures

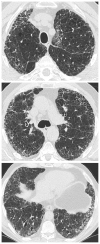


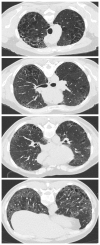



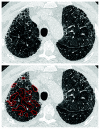


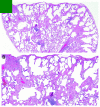


References
-
- Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–93. - PubMed
-
- Cottin V, Cordier JF. The syndrome of combined pulmonary fibrosis and emphysema. Chest. 2009;136:1–2. - PubMed
-
- Wong AW, Liang J, Cottin V, Ryerson CJ. Diagnostic Features in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review. Ann Am Thorac Soc. 2020;17:1333–6. - PubMed
-
- Kawabata Y, Hoshi E, Murai K, et al. Smoking-related changes in the background lung of specimens resected for lung cancer: a semiquantitative study with correlation to postoperative course. Histopathology. 2008;53:707–14. - PubMed
-
- Katzenstein AL. Smoking-related interstitial fibrosis (SRIF): pathologic findings and distinction from other chronic fibrosing lung diseases. J Clin Pathol. 2013;66:882–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

