A digital health program for treatment of urinary incontinence: retrospective review of real-world user data
- PMID: 35969249
- PMCID: PMC10284973
- DOI: 10.1007/s00192-022-05321-3
A digital health program for treatment of urinary incontinence: retrospective review of real-world user data
Erratum in
-
Correction to: A digital health program for treatment of urinary incontinence: retrospective review of real‑world user data.Int Urogynecol J. 2023 Aug;34(8):1993. doi: 10.1007/s00192-023-05596-0. Int Urogynecol J. 2023. PMID: 37368021 Free PMC article. No abstract available.
Abstract
Introduction and hypothesis: To determine the effectiveness of a prescription digital therapeutic (pDTx) in reducing urinary incontinence (UI) symptoms in real-world users.
Methods: This is a retrospective cohort study of real-world data from users of a pDTx designed to guide pelvic floor muscle training(PFMT) between July 1, 2020-December 31, 2021. The primary outcome was UI symptom change as reported via in-app Urogenital Distress Inventory (UDI-6). Included subjects were female, ≥ 18 years with a diagnosis of stress, urgency, or mixed UI who completed the UDI-6 at baseline and 8 weeks. Demographic, symptom, and adherence data were summarized. Paired t-test and Wilcoxon signed rank test were used to analyze change in outcomes from baseline to 8 weeks across adherence and UI diagnosis groups.
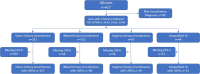
Results: Of 532 women with UI, 265 (50%) met criteria and were included in the analysis. Mean age was 51.2 ± 11.5 years (range 22-84, N = 265). Mean body mass index (BMI) was 27.3 ± 6.2 kg/m2 (range 15.2-46.9, N = 147). Most participants had stress UI (59%) followed by mixed UI (22%), urgency UI/OAB (11%), and unspecified UI (8%). UDI-6 scores improved by 13.90 ± 15.53 (p ≤ 0.001); 62% met or exceeded MCID. Device-reported PFMT adherence was 72% at 4 weeks and 66% at 8 weeks (100% = 14 uses/week). Participants in each diagnosis category reported significant improvement on UDI-6 score from baseline to 8 weeks. No association between UDI-6 score improvement and adherence category, age, BMI, or UI subtype was identified.
Conclusions: This study demonstrates effectiveness of a pDTx in reducing UI symptoms in a real-world setting. Users achieved statistically and clinically significant symptom improvement over an 8-week period.
Keywords: Digital therapeutics; Real-world evidence; Urinary incontinence.
© 2022. The International Urogynecological Association.
Conflict of interest statement
LEK, JLM, and SJP are employees of Renovia Inc. The nature of their employment supports clinical research, scientific exchange, and education and aligns with the aims of this study. They have obtained research, ethics, and compliance training and are CITI certified. MMW has no conflicts of interest to report.
Figures
References
-
- ACOG Practice Bulletin. Urinary incontinence in women. 2018. https://www.acog.org/-/media/Practice-Bulletins/Committee-on-Practice-Bu....
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous