Microfluidic-Derived Detection of Protein-Facilitated Copper Flux Across Lipid Membranes
- PMID: 35969432
- PMCID: PMC9434548
- DOI: 10.1021/acs.analchem.2c02081
Microfluidic-Derived Detection of Protein-Facilitated Copper Flux Across Lipid Membranes
Abstract
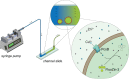
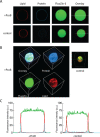
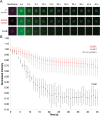
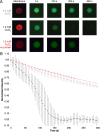
Measurement of protein-facilitated copper flux across biological membranes is a considerable challenge. Here, we demonstrate a straightforward microfluidic-derived approach for visualization and measurement of membranous Cu flux. Giant unilamellar vesicles, reconstituted with the membrane protein of interest, are prepared, surface-immobilized, and assessed using a novel quencher-sensor reporter system for detection of copper. With the aid of a syringe pump, the external buffer is exchanged, enabling consistent and precise exchange of solutes, without causing vesicle rupture or uneven local metal concentrations brought about by rapid mixing. This approach bypasses common issues encountered when studying heavy metal-ion flux, thereby providing a new platform for in vitro studies of metal homeostasis aspects that are critical for all cells, health, and disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Unilamellar vesicle formation and encapsulation by microfluidic jetting.Proc Natl Acad Sci U S A. 2008 Mar 25;105(12):4697-702. doi: 10.1073/pnas.0710875105. Epub 2008 Mar 19. Proc Natl Acad Sci U S A. 2008. PMID: 18353990 Free PMC article.
-
Asymmetric giant lipid vesicle fabrication.Methods Mol Biol. 2015;1232:79-90. doi: 10.1007/978-1-4939-1752-5_7. Methods Mol Biol. 2015. PMID: 25331129
-
Assembly methods for asymmetric lipid and polymer-lipid vesicles.Emerg Top Life Sci. 2022 Dec 22;6(6):609-617. doi: 10.1042/ETLS20220055. Emerg Top Life Sci. 2022. PMID: 36533596 Review.
-
Studying the effects of asymmetry on the bending rigidity of lipid membranes formed by microfluidics.Chem Commun (Camb). 2016 Apr 18;52(30):5277-80. doi: 10.1039/c5cc10307j. Chem Commun (Camb). 2016. PMID: 27001410
-
Elementary Processes and Mechanisms of Interactions of Antimicrobial Peptides with Membranes-Single Giant Unilamellar Vesicle Studies.Adv Exp Med Biol. 2019;1117:17-32. doi: 10.1007/978-981-13-3588-4_3. Adv Exp Med Biol. 2019. PMID: 30980351 Review.
Cited by
-
Emerging Designs and Applications for Biomembrane Biosensors.Annu Rev Anal Chem (Palo Alto Calif). 2024 Jul;17(1):339-366. doi: 10.1146/annurev-anchem-061622-042618. Annu Rev Anal Chem (Palo Alto Calif). 2024. PMID: 39018354 Free PMC article. Review.
-
Functional reconstitution of plant plasma membrane H+-ATPase into giant unilamellar vesicles.Sci Rep. 2025 Mar 12;15(1):8541. doi: 10.1038/s41598-025-92663-9. Sci Rep. 2025. PMID: 40074791 Free PMC article.
-
Lipid vesicle-based molecular robots.Lab Chip. 2024 Feb 27;24(5):996-1029. doi: 10.1039/d3lc00860f. Lab Chip. 2024. PMID: 38239102 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

