Therapeutic Potential of a Water-Soluble Silver-Diclofenac Coordination Polymer on 3D Pancreatic Cancer Spheroids
- PMID: 35969454
- PMCID: PMC9776540
- DOI: 10.1021/acs.jmedchem.2c00535
Therapeutic Potential of a Water-Soluble Silver-Diclofenac Coordination Polymer on 3D Pancreatic Cancer Spheroids
Abstract
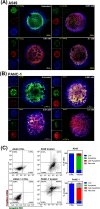
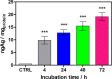
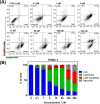
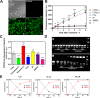
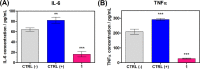
This work describes the traditional wet and green synthetic approaches, structural features, and extensive bioactivity study for a new coordination polymer [Ag(μ-PTA)(Df)(H2O)]n·3nH2O (1) that bears a silver(I) center, a 1,3,5-triaza-phosphaadamantane (PTA) linker, and a nonsteroidal anti-inflammatory drug, diclofenac (Df-). Compared to cisplatin, compound 1 exhibits both anti-inflammatory properties and very remarkable cytotoxicity toward various cancer cell lines with a high value of selectivity index. Additionally, the 3D model representing human pancreas/duct carcinoma (PANC-1) and human lung adenocarcinoma (A549) was designed and applied as a clear proof of the remarkable therapeutic potential of 1. The obtained experimental data indicate that 1 induces an apoptotic pathway via reactive oxygen species generation, targeting mitochondria due to their membrane depolarization. This study broadens a group of bioactive metal-organic networks and highlights the significant potential of such compounds in developing advanced therapeutic solutions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Cai H.; Huang X.-L.; Li D. Biological metal-organic frameworks: Structures, host-guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. 10.1016/j.ccr.2017.12.003. - DOI
-
- Chu Y.; Hou J.; Boyer C.; Richardson J. J.; Liang K.; Xu J. Biomimetic synthesis of coordination network materials: Recent advances in MOFs and MPNs. Appl. Mater. Today 2018, 10, 93–105. 10.1016/j.apmt.2017.12.009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

