RAD51 is a druggable target that sustains replication fork progression upon DNA replication stress
- PMID: 35969531
- PMCID: PMC9377619
- DOI: 10.1371/journal.pone.0266645
RAD51 is a druggable target that sustains replication fork progression upon DNA replication stress
Abstract
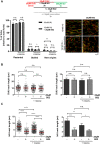
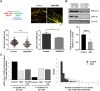
Solving the problems that replication forks encounter when synthesizing DNA is essential to prevent genomic instability. Besides their role in DNA repair in the G2 phase, several homologous recombination proteins, specifically RAD51, have prominent roles in the S phase. Using different cellular models, RAD51 has been shown not only to be present at ongoing and arrested replication forks but also to be involved in nascent DNA protection and replication fork restart. Through pharmacological inhibition, here we study the specific role of RAD51 in the S phase. RAD51 inhibition in non-transformed cell lines did not have a significant effect on replication fork progression under non-perturbed conditions, but when the same cells were subjected to replication stress, RAD51 became necessary to maintain replication fork progression. Notably, the inhibition or depletion of RAD51 did not compromise fork integrity when subjected to hydroxyurea treatment. RAD51 inhibition also did not decrease the ability to restart, but rather compromised fork progression during reinitiation. In agreement with the presence of basal replication stress in human colorectal cancer cells, RAD51 inhibition reduced replication fork speed in these cells and increased γH2Ax foci under control conditions. These alterations could have resulted from the reduced association of DNA polymerase α to chromatin, as observed when inhibiting RAD51. It may be possible to exploit the differential dependence of non-transformed cells versus colorectal cancer cells on RAD51 activity under basal conditions to design new therapies that specifically target cancer cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair.Mol Cell. 2010 Feb 26;37(4):492-502. doi: 10.1016/j.molcel.2010.01.021. Mol Cell. 2010. PMID: 20188668 Free PMC article.
-
NEK8 regulates DNA damage-induced RAD51 foci formation and replication fork protection.Cell Cycle. 2017 Feb 16;16(4):335-347. doi: 10.1080/15384101.2016.1259038. Epub 2016 Nov 28. Cell Cycle. 2017. PMID: 27892797 Free PMC article.
-
Lamin A/C recruits ssDNA protective proteins RPA and RAD51 to stalled replication forks to maintain fork stability.J Biol Chem. 2021 Nov;297(5):101301. doi: 10.1016/j.jbc.2021.101301. Epub 2021 Oct 11. J Biol Chem. 2021. PMID: 34648766 Free PMC article.
-
Moonlighting at replication forks - a new life for homologous recombination proteins BRCA1, BRCA2 and RAD51.FEBS Lett. 2017 Apr;591(8):1083-1100. doi: 10.1002/1873-3468.12556. Epub 2017 Jan 30. FEBS Lett. 2017. PMID: 28079255 Review.
-
Replication Fork Reversal: Players and Guardians.Mol Cell. 2017 Dec 7;68(5):830-833. doi: 10.1016/j.molcel.2017.11.022. Mol Cell. 2017. PMID: 29220651 Free PMC article. Review.
Cited by
-
The timing of pronuclear transfer critically affects the developmental competence and quality of embryos.Mol Hum Reprod. 2024 Jun 26;30(7):gaae024. doi: 10.1093/molehr/gaae024. Mol Hum Reprod. 2024. PMID: 38991843 Free PMC article.

