Early and mid-gestation Zika virus (ZIKV) infection in the olive baboon (Papio anubis) leads to fetal CNS pathology by term gestation
- PMID: 35969617
- PMCID: PMC9410558
- DOI: 10.1371/journal.ppat.1010386
Early and mid-gestation Zika virus (ZIKV) infection in the olive baboon (Papio anubis) leads to fetal CNS pathology by term gestation
Abstract
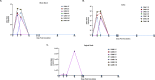
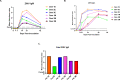
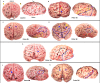
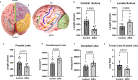
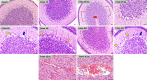
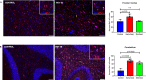
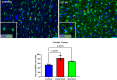
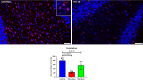
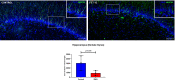
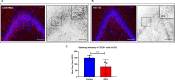
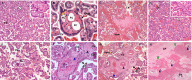
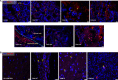
Zika virus (ZIKV) infection in pregnancy can produce catastrophic teratogenic damage to the developing fetus including microcephaly and congenital Zika syndrome (CZS). We previously described fetal CNS pathology occurring by three weeks post-ZIKV inoculation in Olive baboons at mid-gestation, including neuroinflammation, loss of radial glia (RG), RG fibers, neuroprogenitor cells (NPCs) resulting in disrupted NPC migration. In the present study, we explored fetal brain pathologies at term gestation resulting from ZIKV exposure during either first or second trimester in the Olive baboon. In all dams, vRNA in whole blood resolved after 7 days post inoculation (dpi). One first trimester infected dam aborted at 5 dpi. All dams developed IgM and IgG response to ZIKV with ZIKV IgG detected in fetal serum. Placental pathology and inflammation were observed including disruption of syncytiotrophoblast layers, delayed villous maturation, partially or fully thrombosed vessels, calcium mineralization and fibrin deposits. In the uterus, ZIKV was detected in ¾ first trimester but not in second trimester infected dams. While ZIKV was not detected in any fetal tissue at term, all fetuses exhibited varying degrees of neuropathology. Fetal brains from ZIKV inoculated dams exhibited a range of gross brain pathologies including irregularities of the major gyri and sulci of the cerebral cortex and cerebellar pathology. Frontal cortices of ZIKV fetuses showed a general disorganization of the six-layered cortex with degree of disorganization varying among the fetuses from the two groups. Frontal cortices from ZIKV inoculation in the first but not second trimester exhibited increased microglia, and in both trimester ZIKV inoculation, increased astrocyte numbers (white matter). In the cerebellum, increased microglia were observed in fetuses from both first and second trimester inoculation. In first trimester ZIKV inoculation, decreased oligodendrocyte precursor cell populations were observed in fetal cerebellar white matter. In general, our observations are in accordance with those described in human ZIKV infected fetuses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon.PLoS Pathog. 2019 Jan 18;15(1):e1007507. doi: 10.1371/journal.ppat.1007507. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30657788 Free PMC article.
-
Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons.J Virol. 2020 May 18;94(11):e00058-20. doi: 10.1128/JVI.00058-20. Print 2020 May 18. J Virol. 2020. PMID: 32188737 Free PMC article.
-
Zika Virus Infection, Reproductive Organ Targeting, and Semen Transmission in the Male Olive Baboon.J Virol. 2019 Dec 12;94(1):e01434-19. doi: 10.1128/JVI.01434-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597777 Free PMC article.
-
Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection.Arch Gynecol Obstet. 2017 Jun;295(6):1361-1368. doi: 10.1007/s00404-017-4361-5. Epub 2017 Apr 11. Arch Gynecol Obstet. 2017. PMID: 28396992 Free PMC article. Review.
-
Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review.
Cited by
-
The Isolation and In Vitro Differentiation of Primary Fetal Baboon Tracheal Epithelial Cells for the Study of SARS-CoV-2 Host-Virus Interactions.Viruses. 2023 Mar 28;15(4):862. doi: 10.3390/v15040862. Viruses. 2023. PMID: 37112842 Free PMC article.
-
Zika virus and the fetal-maternal interface: deciphering the mechanisms of placental infection and implications for pregnancy outcomes.Emerg Microbes Infect. 2025 Dec;14(1):2532681. doi: 10.1080/22221751.2025.2532681. Epub 2025 Jul 30. Emerg Microbes Infect. 2025. PMID: 40638938 Free PMC article. Review.
-
Comparative Analysis of Two Zika Virus Isolates in a Rhesus Macaque Pregnancy Model.Viruses. 2025 May 27;17(6):762. doi: 10.3390/v17060762. Viruses. 2025. PMID: 40573353 Free PMC article.
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

