Correct use and ease-of-use of placebo ELLIPTA dry-powder inhaler in adult patients with chronic obstructive pulmonary disease
- PMID: 35969632
- PMCID: PMC9377593
- DOI: 10.1371/journal.pone.0273170
Correct use and ease-of-use of placebo ELLIPTA dry-powder inhaler in adult patients with chronic obstructive pulmonary disease
Abstract
Background: Inhaler technique errors are common in chronic obstructive pulmonary disease (COPD) treatment, potentially leading to poor disease management. Our pooled analysis approach assessed correct use and ease-of-use of a placebo ELLIPTA dry-powder inhaler (DPI) in patients with COPD.
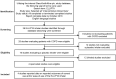
Methods: Adults with COPD from open-label/non-blinded studies evaluating a placebo ELLIPTA DPI and reporting outcomes of correct use (based on the ELLIPTA DPI patient information leaflet [PIL]) and/or ease-of-use were included. Correct use and ease-of use at study end were primary and secondary endpoints, respectively. Data from patients in the placebo ELLIPTA DPI arm of each study were pooled, and the intent-to-treat (ITT) population was used for all analyses.
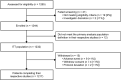
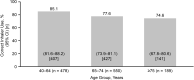
Results: Four placebo ELLIPTA DPI studies, reporting correct use (n = 4) and ease-of-use (n = 2), were included in the analysis. The ITT population comprised 1232 patients (mean age 66.2 years). For the primary endpoint, 80.1% (n = 975/1217) of patients demonstrated correct use at study end (95% confidence interval [CI]: 77.8%-82.3%). For the secondary endpoint, 95.7% (n = 797/833) of patients rated placebo ELLIPTA DPI use "easy"/"very easy" at study end (95% CI: 94.1%-97.0%). Correct use and "easy"/"very easy" user ratings remained high across younger (40-64 years) and older (≥65 years) age groups.
Conclusions: Across age groups, most patients used the placebo ELLIPTA DPI correctly and rated it "easy"/"very easy" to use. Consistent with the Global Initiative for Chronic Obstructive Lung Disease 2021 report, our findings emphasize that proper training and clear instructions on PILs are important for optimal inhaler use.
Conflict of interest statement
RJ, KC, RS, LS, and JR report employment with, and stock/share ownership in, GSK during study conduct. KC is currently employed by AstraZeneca and LS is no longer employed by GSK. TMS received research support from West-Ward Pharmaceuticals, Theravance Biopharma US, Inc., GSK, Pearl Therapeutics, Chiesi, AstraZeneca, Novartis, Boehringer Ingelheim, Forest, Compleware, Evidera, Oncocyte, Teva, Vapotherm, Sunovion, Proterix BioPharma, Seer, and Sanofi. TMS has also received speaker fees from GSK, Mylan Inc./Theravance Biopharma US, Inc., and Sunovion, and consulting fees from Vapotherm. DIB received grant/research/clinical trial support from GSK, Teva, AstraZeneca, Pearl Therapeutics, Novartis, Genentech, Inc., Merck, Boehringer Ingelheim, Amgen, Aimmune, Shire, and Biocryst and consulted/participated in advisory boards for GSK, ALK America, Gerson-Lehman, and Guidepoint Global. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2022 report). 2022. Available from https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

