Nodulisporic acid produces direct activation and positive allosteric modulation of AVR-14B, a glutamate-gated chloride channel from adult Brugia malayi
- PMID: 35969762
- PMCID: PMC9407656
- DOI: 10.1073/pnas.2111932119
Nodulisporic acid produces direct activation and positive allosteric modulation of AVR-14B, a glutamate-gated chloride channel from adult Brugia malayi
Abstract
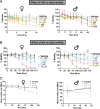
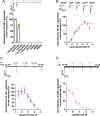
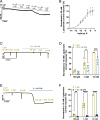
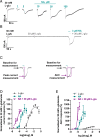
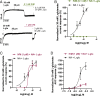
Glutamate-gated chloride channels (GluCls) are unique to invertebrates and are targeted by macrocyclic lactones. In this study, we cloned an AVR-14B GluCl subunit from adult Brugia malayi, a causative agent of lymphatic filariasis in humans. To elucidate this channel's pharmacological properties, we used Xenopus laevis oocytes for expression and performed two-electrode voltage-clamp electrophysiology. The receptor was gated by the natural ligand L-glutamate (effective concentration, 50% [EC50] = 0.4 mM) and ivermectin (IVM; EC50 = 1.8 nM). We also characterized the effects of nodulisporic acid (NA) on Bma-AVR-14B and NA-produced dual effects on the receptor as an agonist and a type II positive allosteric modulator. Here we report characterization of the complex activity of NA on a nematode GluCl. Bma-AVR-14B demonstrated some unique pharmacological characteristics. IVM did not produce potentiation of L-glutamate-mediated responses but instead, reduced the channel's sensitivity for the ligand. Further electrophysiological exploration showed that IVM (at a moderate concentration of 0.1 nM) functioned as an inhibitor of both agonist and positive allosteric modulatory effects of NA. This suggests that IVM and NA share a complex interaction. The pharmacological properties of Bma-AVR-14B indicate that the channel is an important target of IVM and NA. In addition, the unique electrophysiological characteristics of Bma-AVR-14B could explain the observed variation in drug sensitivities of various nematode parasites. We have also shown the inhibitory effects of IVM and NA on adult worm motility using Worminator. RNA interference (RNAi) knockdown suggests that AVR-14 plays a role in influencing locomotion in B. malayi.
Keywords: AVR-14B; Brugia malayi; filarial nematode; glutamate-gated chloride channels; nodulisporic acid.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Nodulisporic acid opens insect glutamate-gated chloride channels: identification of a new high affinity modulator.Biochemistry. 2000 May 9;39(18):5543-54. doi: 10.1021/bi992943i. Biochemistry. 2000. PMID: 10820028
-
Electrophysiological characterization of ivermectin triple actions on Musca chloride channels gated by l-glutamic acid and γ-aminobutyric acid.Insect Biochem Mol Biol. 2016 Oct;77:78-86. doi: 10.1016/j.ibmb.2016.08.005. Epub 2016 Aug 16. Insect Biochem Mol Biol. 2016. PMID: 27543424
-
Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi.Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):20120-5. doi: 10.1073/pnas.1011983107. Epub 2010 Nov 1. Proc Natl Acad Sci U S A. 2010. PMID: 21041637 Free PMC article.
-
Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics.Parasitology. 2005;131 Suppl:S85-95. doi: 10.1017/S0031182005008218. Parasitology. 2005. PMID: 16569295 Review.
-
[Pharmacological effects of ivermectin, an antiparasitic agent for intestinal strongyloidiasis: its mode of action and clinical efficacy].Nihon Yakurigaku Zasshi. 2003 Dec;122(6):527-38. doi: 10.1254/fpj.122.527. Nihon Yakurigaku Zasshi. 2003. PMID: 14639007 Review. Japanese.
Cited by
-
Biosynthesis of Nodulisporic Acids: A Multifunctional Monooxygenase Delivers a Complex and Highly Branched Array.Angew Chem Int Ed Engl. 2022 Dec 5;61(49):e202213364. doi: 10.1002/anie.202213364. Epub 2022 Nov 9. Angew Chem Int Ed Engl. 2022. PMID: 36199176 Free PMC article.
-
Resolving the origins of secretory products and anthelmintic responses in a human parasitic nematode at single-cell resolution.Elife. 2023 Jun 15;12:e83100. doi: 10.7554/eLife.83100. Elife. 2023. PMID: 37318129 Free PMC article.
References
-
- Cully D. F., Wilkinson H., Vassilatis D. K., Etter A., Arena J. P., Molecular biology and electrophysiology of glutamate-gated chloride channels of invertebrates. Parasitology 113 (suppl.), S191–S200 (1996). - PubMed
-
- Cleland T. A., Inhibitory glutamate receptor channels. Mol. Neurobiol. 13, 97–136 (1996). - PubMed
-
- Vassilatis D. K., et al. , Evolutionary relationship of the ligand-gated ion channels and the avermectin-sensitive, glutamate-gated chloride channels. J. Mol. Evol. 44, 501–508 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

