Integrated AlphaFold2 and DEER investigation of the conformational dynamics of a pH-dependent APC antiporter
- PMID: 35969794
- PMCID: PMC9407458
- DOI: 10.1073/pnas.2206129119
Integrated AlphaFold2 and DEER investigation of the conformational dynamics of a pH-dependent APC antiporter
Abstract
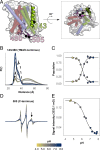
The Amino Acid-Polyamine-Organocation (APC) transporter GadC contributes to the survival of pathogenic bacteria under extreme acid stress by exchanging extracellular glutamate for intracellular γ-aminobutyric acid (GABA). Its structure, determined in an inward-facing conformation at alkaline pH, consists of the canonical LeuT-fold with a conserved five-helix inverted repeat, thereby resembling functionally divergent transporters such as the serotonin transporter SERT and the glucose-sodium symporter SGLT1. However, despite this structural similarity, it is unclear if the conformational dynamics of antiporters such as GadC follow the blueprint of these or other LeuT-fold transporters. Here, we used double electron-electron resonance (DEER) spectroscopy to monitor the conformational dynamics of GadC in lipid bilayers in response to acidification and substrate binding. To guide experimental design and facilitate the interpretation of the DEER data, we generated an ensemble of structural models in multiple conformations using a recently introduced modification of AlphaFold2 . Our experimental results reveal acid-induced conformational changes that dislodge the Cterminus from the permeation pathway coupled with rearrangement of helices that enables isomerization between inward- and outward-facing states. The substrate glutamate, but not GABA, modulates the dynamics of an extracellular thin gate without shifting the equilibrium between inward- and outward-facing conformations. In addition to introducing an integrated methodology for probing transporter conformational dynamics, the congruence of the DEER data with patterns of structural rearrangements deduced from ensembles of AlphaFold2 models illuminates the conformational cycle of GadC underpinning transport and exposes yet another example of the divergence between the dynamics of different families in the LeuT-fold.
Keywords: acid resistance; amino acid transport; membrane protein biophysics; structure prediction.
Conflict of interest statement
The authors declare no competing interest.
Figures






Similar articles
-
Alternating access of a bacterial homolog of neurotransmitter: sodium symporters determined from AlphaFold2 ensembles and DEER spectroscopy.Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2406063121. doi: 10.1073/pnas.2406063121. Epub 2024 Sep 20. Proc Natl Acad Sci U S A. 2024. PMID: 39302996 Free PMC article.
-
Conformational cycle and ion-coupling mechanism of the Na+/hydantoin transporter Mhp1.Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14752-7. doi: 10.1073/pnas.1410431111. Epub 2014 Sep 29. Proc Natl Acad Sci U S A. 2014. PMID: 25267652 Free PMC article.
-
Function and Regulation of Acid Resistance Antiporters.J Membr Biol. 2019 Oct;252(4-5):465-481. doi: 10.1007/s00232-019-00073-6. Epub 2019 Jun 25. J Membr Biol. 2019. PMID: 31240358
-
Principles of Alternating Access in LeuT-fold Transporters: Commonalities and Divergences.J Mol Biol. 2022 Oct 15;434(19):167746. doi: 10.1016/j.jmb.2022.167746. Epub 2022 Jul 16. J Mol Biol. 2022. PMID: 35843285 Review.
-
Identifying and quantitating conformational exchange in membrane proteins using site-directed spin labeling.Acc Chem Res. 2014 Oct 21;47(10):3102-9. doi: 10.1021/ar500228s. Epub 2014 Aug 25. Acc Chem Res. 2014. PMID: 25152957 Free PMC article. Review.
Cited by
-
Molecular basis of pyruvate transport and inhibition of the human mitochondrial pyruvate carrier.Sci Adv. 2025 Apr 18;11(16):eadw1489. doi: 10.1126/sciadv.adw1489. Epub 2025 Apr 18. Sci Adv. 2025. PMID: 40249800 Free PMC article.
-
Protein Modeling with DEER Spectroscopy.Annu Rev Biophys. 2025 May;54(1):35-57. doi: 10.1146/annurev-biophys-030524-013431. Epub 2024 Dec 17. Annu Rev Biophys. 2025. PMID: 39689263 Free PMC article. Review.
-
Darobactin B Stabilises a Lateral-Closed Conformation of the BAM Complex in E. coli Cells.Angew Chem Weinheim Bergstr Ger. 2023 Aug 21;135(34):e202218783. doi: 10.1002/ange.202218783. Epub 2023 May 31. Angew Chem Weinheim Bergstr Ger. 2023. PMID: 38515502 Free PMC article.
-
Deep learning for protein structure prediction and design-progress and applications.Mol Syst Biol. 2024 Mar;20(3):162-169. doi: 10.1038/s44320-024-00016-x. Epub 2024 Jan 30. Mol Syst Biol. 2024. PMID: 38291232 Free PMC article. Review.
-
Insights into mechanisms of MALT1 allostery from NMR and AlphaFold dynamic analyses.Commun Biol. 2024 Jul 16;7(1):868. doi: 10.1038/s42003-024-06558-y. Commun Biol. 2024. PMID: 39014105 Free PMC article.
References
-
- Västermark Å., Saier M. H. Jr., Evolutionary relationship between 5 + 5 and 7 + 7 inverted repeat folds within the amino acid-polyamine-organocation superfamily. Proteins 82, 336–346 (2014). - PubMed
-
- Kandasamy P., Gyim-esi G., Kanai Y., Hediger M. A., Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 43, 752–789 (2018). - PubMed
-
- Fotiadis D., Kanai Y., Palacín M., The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 34, 139–158 (2013). - PubMed
-
- Jack D. L., Paulsen I. T., Saier M. H., The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology (Reading) 146, 1797–1814 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

