Antiviral Profiling of C-18- or C-19-Functionalized Semisynthetic Abietane Diterpenoids
- PMID: 35969814
- PMCID: PMC9425435
- DOI: 10.1021/acs.jnatprod.2c00464
Antiviral Profiling of C-18- or C-19-Functionalized Semisynthetic Abietane Diterpenoids
Abstract
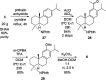
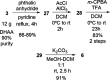
Viral infections affect several million patients annually. Although hundreds of viruses are known to be pathogenic, only a few can be treated in the clinic with available antiviral drugs. Naturally based pharmacotherapy may be a proper alternative for treating viral diseases. Several natural and semisynthetic abietane-type diterpenoids have shown important antiviral activities. In this study, a biological evaluation of a number of either C-18- or C-19-functionalized known semisynthetic abietanes against Zika virus, Dengue virus, Herpes virus simplex type 1, and Chikungunya virus are reported. Semisynthetic abietane ferruginol and its analogue 18-(phthalimid-2-yl)ferruginol displayed broad-spectrum antiviral properties. The scale-up synthesis of this analogue has been optimized for further studies and development. This molecule displayed an EC50 between 5.0 and 10.0 μM against Colombian Zika virus strains and EC50 = 9.8 μM against Chikungunya virus. Knowing that this ferruginol analogue is also active against Dengue virus type 2 (EC50 = 1.4 μM, DENV-2), we can conclude that this compound is a promising broad-spectrum antiviral agent paving the way for the development of novel antivirals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

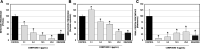
Similar articles
-
Anti-Zika virus activity of several abietane-type ferruginol analogues.Rev Inst Med Trop Sao Paulo. 2020 Dec 7;62:e97. doi: 10.1590/S1678-9946202062097. eCollection 2020. Rev Inst Med Trop Sao Paulo. 2020. PMID: 33295481 Free PMC article.
-
Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine.Eur J Med Chem. 2016 Jan 27;108:79-88. doi: 10.1016/j.ejmech.2015.11.009. Epub 2015 Nov 11. Eur J Med Chem. 2016. PMID: 26638041 Free PMC article.
-
Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues.Viruses. 2023 Jun 9;15(6):1342. doi: 10.3390/v15061342. Viruses. 2023. PMID: 37376641 Free PMC article.
-
Host Cell Targets for Unconventional Antivirals against RNA Viruses.Viruses. 2023 Mar 17;15(3):776. doi: 10.3390/v15030776. Viruses. 2023. PMID: 36992484 Free PMC article. Review.
-
Endemic and Indigenous Plants from Mascarene Islands with Antiviral Propensities.Curr Drug Targets. 2022;23(1):72-86. doi: 10.2174/1389450122666210824143910. Curr Drug Targets. 2022. PMID: 34431460 Review.
Cited by
-
Synthesis and Evaluation of Antimicrobial Activity of the Rearranged Abietane Prattinin A and Its Synthetic Derivatives.Molecules. 2024 Jan 30;29(3):650. doi: 10.3390/molecules29030650. Molecules. 2024. PMID: 38338393 Free PMC article.
-
Synergistic In Vitro Antiviral Effect of Combinations of Ivermectin, Essential Oils, and 18-(Phthalimid-2-yl)ferruginol against Arboviruses and Herpesvirus.Pharmaceuticals (Basel). 2023 Nov 13;16(11):1602. doi: 10.3390/ph16111602. Pharmaceuticals (Basel). 2023. PMID: 38004467 Free PMC article.
-
Discovery of Novel Bioactive Tanshinones and Carnosol Analogues against Breast Cancer.Cancers (Basel). 2023 Feb 19;15(4):1318. doi: 10.3390/cancers15041318. Cancers (Basel). 2023. PMID: 36831660 Free PMC article.
-
In Vitro Effect of Ferruginol, Tanshinone, and Carnosol Analogues on the Proliferation of Three Breast Cancer Cell Lines.Molecules. 2025 Jun 10;30(12):2529. doi: 10.3390/molecules30122529. Molecules. 2025. PMID: 40572493 Free PMC article.
-
In Vitro Cytotoxic Effects of Ferruginol Analogues in Sk-MEL28 Human Melanoma Cells.Int J Mol Sci. 2023 Nov 14;24(22):16322. doi: 10.3390/ijms242216322. Int J Mol Sci. 2023. PMID: 38003511 Free PMC article.
References
-
- Pan American Health Organization. Tool for the diagnosis and care of patients with suspected arboviral diseases; Pan American Health Organization: Washington, D.C., 2017. https://iris.paho.org/handle/10665.2/33895.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

