Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease
- PMID: 35969834
- PMCID: PMC9837450
- DOI: 10.1182/blood.2021015352
Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease
Erratum in
-
Burgos da Silva M, Ponce DM, Dai A, et al. Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood. 2022;140(22):2385-2397.Blood. 2023 Mar 9;141(10):1234. doi: 10.1182/blood.2023019750. Blood. 2023. PMID: 36893004 Free PMC article. No abstract available.
Abstract
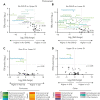
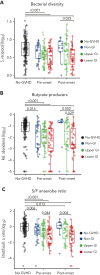
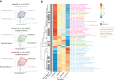
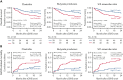
Following allogeneic hematopoietic cell transplantation (allo-HCT), the gastrointestinal (GI) tract is frequently affected by acute graft-versus-host disease (aGVHD), the pathophysiology of which is associated with a dysbiotic microbiome. Since microbial composition varies along the length of the GI tract, the authors hypothesized that microbiome features correlate with the pattern of organ involvement after allo-HCT. We evaluated 266 allo-HCT recipients from whom 1303 stool samples were profiled by 16S ribosomal gene sequencing. Patients were classified according to which organs were affected by aGVHD. In the 20 days prior to disease onset, GVHD patients had lower abundances of members of the class Clostridia, lower counts of butyrate producers, and lower ratios of strict-to-facultative (S/F) anaerobic bacteria compared with allograft recipients who were free of GVHD. GI GVHD patients showed significant reduction in microbial diversity preonset. Patients with lower GI aGVHD had lower S/F anaerobe ratios compared with those with isolated upper GI aGVHD. In the 20 days after disease onset, dysbiosis was observed only in GVHD patients with GI involvement, particularly those with lower-tract disease. Importantly, Clostridial and butyrate-producer abundance as well as S/F anaerobe ratio were predictors of longer overall survival; higher abundance of butyrate producers and higher S/F anaerobe ratio were associated with decreased risk of GVHD-related death. These findings suggest that the intestinal microbiome can serve as a biomarker for outcomes of allo-HCT patients with GVHD.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.M.P. has served as advisory board member for Evive Biotechnology (Shanghai) Ltd (formerly Generon [Shanghai] Corporation Ltd); has consulted, received honorarium from, or particpated in advisory boards for Kadmon Corporation/Sanofi, CareDx, Ceramedix, and Incyte; has received research support from Incyte. A.L.C.G. reports equity, salary and is employed by Xbiome Co. R.S. received consultancy fees from Medexus and MyBiotics. M.-A.P. reports honoraria from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. J.U.P. reports research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics and consulting fees from DaVolterra, CSL Behring, and MaaT Pharma. He serves on an advisory board of and holds equity in Postbiotics Plus Research. He has filed intellectual property applications related to the microbiome (reference numbers 62/843,849, 62/977,908, and 15/756,845). M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaxoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (Spouse), Synthekine (Spouse), Beigene (Spouse), Kite (Spouse); he has IP Licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization). Memorial Sloan Kettering Cancer Center (MSK) has financial interests relative to Seres Therapeutics. The remaining authors declare no competing financial interests.
Figures



Comment in
-
GVHD prediction based on the microbiome.Blood. 2022 Dec 1;140(22):2313-2314. doi: 10.1182/blood.2022017462. Blood. 2022. PMID: 36454594 No abstract available.
References
-
- Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

