Clinical Practice Guidelines From the Association for the Advancement of Blood and Biotherapies (AABB): COVID-19 Convalescent Plasma
- PMID: 35969859
- PMCID: PMC9450870
- DOI: 10.7326/M22-1079
Clinical Practice Guidelines From the Association for the Advancement of Blood and Biotherapies (AABB): COVID-19 Convalescent Plasma
Abstract
Description: Coronavirus disease 2019 convalescent plasma (CCP) has emerged as a potential treatment of COVID-19. However, meta-analysis data and recommendations are limited. The Association for the Advancement of Blood and Biotherapies (AABB) developed clinical practice guidelines for the appropriate use of CCP.
Methods: These guidelines are based on 2 living systematic reviews of randomized controlled trials (RCTs) evaluating CCP from 1 January 2019 to 26 January 2022. There were 33 RCTs assessing 21 916 participants. The results were summarized using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) method. An expert panel reviewed the data using the GRADE framework to formulate recommendations.
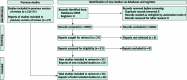
Recommendation 1 (outpatient): The AABB suggests CCP transfusion in addition to the usual standard of care for outpatients with COVID-19 who are at high risk for disease progression (weak recommendation, moderate-certainty evidence).
Recommendation 2 (inpatient): The AABB recommends against CCP transfusion for unselected hospitalized persons with moderate or severe disease (strong recommendation, high-certainty evidence). This recommendation does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2.
Recommendation 3 (inpatient): The AABB suggests CCP transfusion in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies detected at admission (weak recommendation, low-certainty evidence).
Recommendation 4 (inpatient): The AABB suggests CCP transfusion in addition to the usual standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression (weak recommendation, low-certainty evidence).
Recommendation 5 (prophylaxis): The AABB suggests against prophylactic CCP transfusion for uninfected persons with close contact exposure to a person with COVID-19 (weak recommendation, low-certainty evidence).
Good clinical practice statement: CCP is most effective when transfused with high neutralizing titers to infected patients early after symptom onset.
Conflict of interest statement
Figures





Comment in
-
The Fast and the Furious: Chasing a Clinical Niche for COVID-19 Convalescent Plasma.Ann Intern Med. 2022 Sep;175(9):1332-1334. doi: 10.7326/M22-2329. Epub 2022 Aug 16. Ann Intern Med. 2022. PMID: 35969864 Free PMC article.
References
-
- Luke TC, Kilbane EM, Jackson JL, et al. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment. Ann Intern Med. 2006;145:599-609. [PMID: ] - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
