An Assessment of a Rapid SARS-CoV-2 Antigen Test in Bangladesh
- PMID: 35970285
- PMCID: PMC9651516
- DOI: 10.4269/ajtmh.22-0068
An Assessment of a Rapid SARS-CoV-2 Antigen Test in Bangladesh
Abstract
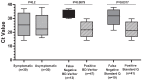
Early detection of SARS-CoV-2 infection is crucial to prevent its spread. This study aimed to document test sensitivity/specificity, correlation with cycle threshold value from polymerase chain reaction (PCR), fitness-for-use in different populations and settings, and user perspectives that could inform large-scale implementation. In this study, we evaluated the performance of a rapid antigen detection test, BD Veritor, and compared this (and another rapid test, Standard Q) against reverse transcription PCR (RT-PCR) in terms of sensitivity and specificity in 130 symptomatic and 130 asymptomatic adults. In addition, we evaluated the suitability and ease of use of the BD Veritor test in a subsample of study participants (n = 42) and implementers (n = 5). At 95% confidence interval, the sensitivity of the BD Veritor and Standard Q test were 70% and 63% in symptomatic and 87% and 73% in asymptomatic individuals, respectively, regarding positive SARS-CoV-2 RT-PCR results. Overall, the BD Veritor test was 78% sensitive and 99.5% specific compared with RT-PCR irrespective of the cycle threshold. This warrants large field evaluation as well as use of the rapid antigen test for quick assessment of SARS-CoV-2 for containment of epidemics in the country.
Figures
References
-
- Johansson MA. et al., 2020. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: Symptom monitoring, quarantine, and testing. medRxiv. Available at: https://www.medrxiv.org/content/10.1101/2020.11.23.20237412v1. Accessed June 15, 2021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

