Sex-Dependent Hepatoprotective Role of IL-22 Receptor Signaling in Non-Alcoholic Fatty Liver Disease-Related Fibrosis
- PMID: 35970323
- PMCID: PMC9596743
- DOI: 10.1016/j.jcmgh.2022.08.001
Sex-Dependent Hepatoprotective Role of IL-22 Receptor Signaling in Non-Alcoholic Fatty Liver Disease-Related Fibrosis
Abstract
Background & aims: Nonalcoholic fatty liver disease (NAFLD) is a major health problem with complex pathogenesis. Although sex differences in NAFLD pathogenesis have been reported, the mechanisms underlying such differences remain understudied. Interleukin (IL)22 is a pleiotropic cytokine with both protective and/or pathogenic effects during liver injury. IL22 was shown to be hepatoprotective in NAFLD-related liver injury. However, these studies relied primarily on exogenous administration of IL22 and did not examine the sex-dependent effect of IL22. Here, we sought to characterize the role of endogenous IL22-receptor signaling during NAFLD-induced liver injury in males and females.
Methods: We used immunofluorescence, flow cytometry, histopathologic assessment, and gene expression analysis to examine IL22 production and characterize the intrahepatic immune landscape in human subjects with NAFLD (n = 20; 11 men and 9 women) and in an in vivo Western high-fat diet-induced NAFLD model in IL22RA knock out mice and their wild-type littermates.
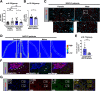
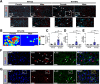
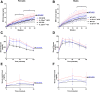
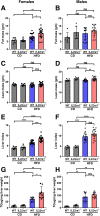
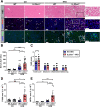
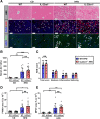
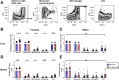
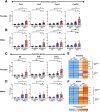
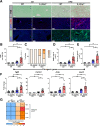
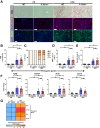
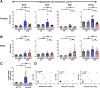
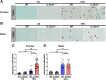
Results: Examination of publicly available data sets from 2 cohorts with NAFLD showed increased hepatic IL22 gene expression in females compared with males. Furthermore, our immunofluorescence analysis of liver sections from NAFLD subjects (n = 20) showed increased infiltration of IL22-producing cells in females. Similarly, IL22-producing cells were increased in wild-type female mice with NAFLD and the hepatic IL22/IL22 binding protein messenger RNA ratio correlated with expression of anti-apoptosis genes. The lack of endogenous IL22-receptor signaling (IL22RA knockout) led to exacerbated liver damage, inflammation, apoptosis, and liver fibrosis in female, but not male, mice with NAFLD.
Conclusions: Our data suggest a sex-dependent hepatoprotective antiapoptotic effect of IL22-receptor signaling during NAFLD-related liver injury in females.
Keywords: IL22 receptor signaling; IL22BP; NAFLD; liver fibrosis.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Formyl peptide receptor 2 determines sex-specific differences in the progression of nonalcoholic fatty liver disease and steatohepatitis.Nat Commun. 2022 Jan 31;13(1):578. doi: 10.1038/s41467-022-28138-6. Nat Commun. 2022. PMID: 35102146 Free PMC article.
-
Sex-based differences in natural killer T cell-mediated protection against diet-induced steatohepatitis in Balb/c mice.Biol Sex Differ. 2023 Nov 14;14(1):85. doi: 10.1186/s13293-023-00569-w. Biol Sex Differ. 2023. PMID: 37964320 Free PMC article.
-
Dysfunction of estrogen-related receptor alpha-dependent hepatic VLDL secretion contributes to sex disparity in NAFLD/NASH development.Theranostics. 2020 Aug 29;10(24):10874-10891. doi: 10.7150/thno.47037. eCollection 2020. Theranostics. 2020. PMID: 33042259 Free PMC article.
-
Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis.World J Gastroenterol. 2016 Nov 28;22(44):9706-9717. doi: 10.3748/wjg.v22.i44.9706. World J Gastroenterol. 2016. PMID: 27956794 Free PMC article. Review.
-
NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk.Adv Ther. 2017 Jun;34(6):1291-1326. doi: 10.1007/s12325-017-0556-1. Epub 2017 May 19. Adv Ther. 2017. PMID: 28526997 Free PMC article. Review.
Cited by
-
Hemopexin and HO-1 induction during acute colitis in mice is dependent on interleukin-22.Front Immunol. 2025 Jul 28;16:1614466. doi: 10.3389/fimmu.2025.1614466. eCollection 2025. Front Immunol. 2025. PMID: 40791589 Free PMC article.
-
Secreted proteins in treating metabolic dysfunction-associated steatotic liver disease: from bench towards bedside.Protein Cell. 2025 Aug 7;16(8):641-666. doi: 10.1093/procel/pwaf027. Protein Cell. 2025. PMID: 40243151 Free PMC article.
-
Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives.Cells. 2023 Aug 22;12(17):2129. doi: 10.3390/cells12172129. Cells. 2023. PMID: 37681858 Free PMC article. Review.
-
Novel RORγt inverse agonists limit IL-17-mediated liver inflammation and fibrosis.J Immunol. 2025 Jun 1;214(6):1321-1331. doi: 10.1093/jimmun/vkaf014. J Immunol. 2025. PMID: 40073158
-
The Effect of Sex-Specific Differences on IL-10-/- Mouse Colitis Phenotype and Microbiota.Int J Mol Sci. 2023 Jun 20;24(12):10364. doi: 10.3390/ijms241210364. Int J Mol Sci. 2023. PMID: 37373511 Free PMC article.
References
-
- Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

