Fast Cysteine Bioconjugation Chemistry
- PMID: 35970770
- PMCID: PMC9701160
- DOI: 10.1002/chem.202201843
Fast Cysteine Bioconjugation Chemistry
Abstract
Cysteine bioconjugation serves as a powerful tool in biological research and has been widely used for chemical modification of proteins, constructing antibody-drug conjugates, and enabling cell imaging studies. Cysteine conjugation reactions with fast kinetics and exquisite selectivity have been under heavy pursuit as they would allow clean protein modification with just stoichiometric amounts of reagents, which minimizes side reactions, simplifies purification and broadens functional group tolerance. In this concept, we summarize the recent advances in fast cysteine bioconjugation, and discuss the mechanism and chemical principles that underlie the high efficiencies of the newly developed cysteine reactive reagents.
Keywords: N-terminal cysteine; cysteine bioconjugation; fast kinetics; protein modification; reaction rate constant.
© 2022 Wiley-VCH GmbH.
Figures







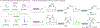
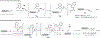
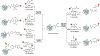

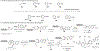

Similar articles
-
Stoichiometric and irreversible cysteine-selective protein modification using carbonylacrylic reagents.Nat Commun. 2016 Oct 26;7:13128. doi: 10.1038/ncomms13128. Nat Commun. 2016. PMID: 27782215 Free PMC article.
-
Site-Specifically Labeled Immunoconjugates for Molecular Imaging--Part 1: Cysteine Residues and Glycans.Mol Imaging Biol. 2016 Feb;18(1):1-17. doi: 10.1007/s11307-015-0919-4. Mol Imaging Biol. 2016. PMID: 26754790 Free PMC article. Review.
-
Site-selective lysine conjugation methods and applications towards antibody-drug conjugates.Chem Commun (Camb). 2021 Oct 14;57(82):10689-10702. doi: 10.1039/d1cc03976h. Chem Commun (Camb). 2021. PMID: 34570125 Free PMC article. Review.
-
Tyrosine Conjugation Methods for Protein Labelling.Chemistry. 2020 Nov 11;26(63):14257-14269. doi: 10.1002/chem.202001992. Epub 2020 Sep 23. Chemistry. 2020. PMID: 32538529 Review.
-
Silicon-Containing Thiol-Specific Bioconjugating Reagent.J Am Chem Soc. 2024 Jan 24;146(3):1776-1782. doi: 10.1021/jacs.3c12050. Epub 2024 Jan 10. J Am Chem Soc. 2024. PMID: 38198597
Cited by
-
NSPs: chromogenic linkers for fast, selective, and irreversible cysteine modification.Chem Sci. 2024 Jun 14;15(28):10997-11004. doi: 10.1039/d4sc01710b. eCollection 2024 Jul 17. Chem Sci. 2024. PMID: 39027294 Free PMC article.
-
Heterotelechelic Organometallic PEG Reagents Enable Modular Access to Complex Bioconjugates.ACS Macro Lett. 2024 Nov 19;13(11):1551-1557. doi: 10.1021/acsmacrolett.4c00588. Epub 2024 Oct 31. ACS Macro Lett. 2024. PMID: 39480964
-
Diazaborine-Mediated Bicyclization of Native Peptides with Inducible Reversibility.Org Lett. 2023 Jun 23;25(24):4489-4492. doi: 10.1021/acs.orglett.3c01496. Epub 2023 Jun 12. Org Lett. 2023. PMID: 37306633 Free PMC article.
-
Spontaneous Self-Organized Order Emerging From Intrinsically Disordered Protein Polymers.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2025 Jan-Feb;17(1):e70003. doi: 10.1002/wnan.70003. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2025. PMID: 39950263 Free PMC article. Review.
-
Late-Stage Aromatic C-H Bond Functionalization for Cysteine/Selenocysteine Bioconjugation.J Am Chem Soc. 2025 Sep 3;147(35):31811-31820. doi: 10.1021/jacs.5c08936. Epub 2025 Aug 19. J Am Chem Soc. 2025. PMID: 40828607 Free PMC article.
References
-
- Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND, J. Nutr 2004, 134, 489–492. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

