Chalcogen bond-guided conformational isomerization enables catalytic dynamic kinetic resolution of sulfoxides
- PMID: 35970848
- PMCID: PMC9378665
- DOI: 10.1038/s41467-022-32428-4
Chalcogen bond-guided conformational isomerization enables catalytic dynamic kinetic resolution of sulfoxides
Abstract
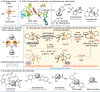
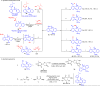
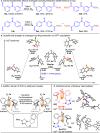
Conformational isomerization can be guided by weak interactions such as chalcogen bonding (ChB) interactions. Here we report a catalytic strategy for asymmetric access to chiral sulfoxides by employing conformational isomerization and chalcogen bonding interactions. The reaction involves a sulfoxide bearing two aldehyde moieties as the substrate that, according to structural analysis and DFT calculations, exists as a racemic mixture due to the presence of an intramolecular chalcogen bond. This chalcogen bond formed between aldehyde (oxygen atom) and sulfoxide (sulfur atom), induces a conformational locking effect, thus making the symmetric sulfoxide as a racemate. In the presence of N-heterocyclic carbene (NHC) as catalyst, the aldehyde moiety activated by the chalcogen bond selectively reacts with an alcohol to afford the corresponding chiral sulfoxide products with excellent optical purities. This reaction involves a dynamic kinetic resolution (DKR) process enabled by conformational locking and facile isomerization by chalcogen bonding interactions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Carbene-Catalyzed and Pnictogen Bond-Assisted Access to PIII-Stereogenic Compounds.Angew Chem Int Ed Engl. 2024 Jul 8;63(28):e202404477. doi: 10.1002/anie.202404477. Epub 2024 Jun 11. Angew Chem Int Ed Engl. 2024. PMID: 38669345
-
Syntheses, and Structural and Physical Properties of Axially Chiral Biaryl Dicarboxylic Acids Bearing Chalcogen Atoms.Chem Pharm Bull (Tokyo). 2022;70(9):605-615. doi: 10.1248/cpb.c22-00217. Chem Pharm Bull (Tokyo). 2022. PMID: 36047231
-
Modeling substrate- and inhibitor-bound forms of liver alcohol dehydrogenase: chemistry of mononuclear nitrogen/sulfur-ligated zinc alcohol, formamide, and sulfoxide complexes.Inorg Chem. 2002 Sep 23;41(19):4872-87. doi: 10.1021/ic0255609. Inorg Chem. 2002. PMID: 12230391
-
Chalcogen bonding catalysis.Chem Soc Rev. 2024 Jan 22;53(2):586-605. doi: 10.1039/d3cs00503h. Chem Soc Rev. 2024. PMID: 38059482 Review.
-
Development of chiral sulfoxide ligands for asymmetric catalysis.Angew Chem Int Ed Engl. 2015 Apr 20;54(17):5026-43. doi: 10.1002/anie.201411073. Epub 2015 Mar 20. Angew Chem Int Ed Engl. 2015. PMID: 25801825 Review.
Cited by
-
Columnar Mesomorphism in a Methylthio-Decorated Triindole for Enhanced Charge Transport.ACS Appl Electron Mater. 2024 Jun 11;6(6):4709-4717. doi: 10.1021/acsaelm.4c00693. eCollection 2024 Jun 25. ACS Appl Electron Mater. 2024. PMID: 38947954 Free PMC article.
-
Enabling Construction of Boron-Stereogenic Formyl BODIPYs via N‑Heterocyclic Carbene-Catalyzed Enantioselective Esterification.JACS Au. 2025 May 16;5(6):2837-2848. doi: 10.1021/jacsau.5c00425. eCollection 2025 Jun 23. JACS Au. 2025. PMID: 40575290 Free PMC article.
-
Effect of Chalcogen Interaction on the Structure of Methine-Bridged Trichalcogenophenes.Chemistry. 2025 Jul 17;31(40):e202501123. doi: 10.1002/chem.202501123. Epub 2025 Jun 4. Chemistry. 2025. PMID: 40419448 Free PMC article.
-
Intramolecular chalcogen bonding activated SuFEx click chemistry for efficient organic-inorganic linking.Nat Commun. 2024 Aug 10;15(1):6849. doi: 10.1038/s41467-024-50922-9. Nat Commun. 2024. PMID: 39127764 Free PMC article.
-
A molecular descriptor of intramolecular noncovalent interaction for regulating optoelectronic properties of organic semiconductors.Nat Commun. 2023 May 1;14(1):2500. doi: 10.1038/s41467-023-38078-4. Nat Commun. 2023. PMID: 37127693 Free PMC article.
References
-
- Desiraju GR, et al. Definition of the halogen bond. Pure. Appl. Chem. 2013;85:1711–1713. doi: 10.1351/PAC-REC-12-05-10. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

