PKR and TLR3 trigger distinct signals that coordinate the induction of antiviral apoptosis
- PMID: 35970851
- PMCID: PMC9378677
- DOI: 10.1038/s41419-022-05101-3
PKR and TLR3 trigger distinct signals that coordinate the induction of antiviral apoptosis
Erratum in
-
Correction: PKR and TLR3 trigger distinct signals that coordinate the induction of antiviral apoptosis.Cell Death Dis. 2022 Sep 19;13(9):798. doi: 10.1038/s41419-022-05227-4. Cell Death Dis. 2022. PMID: 36123340 Free PMC article. No abstract available.
Abstract
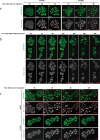
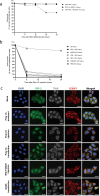
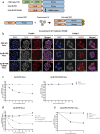
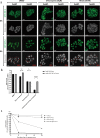
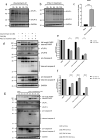
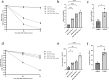
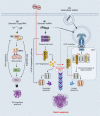
RIG-I-like receptors (RLRs), protein kinase R (PKR), and endosomal Toll-like receptor 3 (TLR3) sense viral non-self RNA and are involved in cell fate determination. However, the mechanisms by which intracellular RNA induces apoptosis, particularly the role of each RNA sensor, remain unclear. We performed cytoplasmic injections of different types of RNA and elucidated the molecular mechanisms underlying viral dsRNA-induced apoptosis. The results obtained revealed that short 5'-triphosphate dsRNA, the sole ligand of RIG-I, induced slow apoptosis in a fraction of cells depending on IRF-3 transcriptional activity and IFN-I production. However, intracellular long dsRNA was sensed by PKR and TLR3, which activate distinct signals, and synergistically induced rapid apoptosis. PKR essentially induced translational arrest, resulting in reduced levels of cellular FLICE-like inhibitory protein and functioned in the TLR3/TRIF-dependent activation of caspase 8. The present results demonstrated that PKR and TLR3 were both essential for inducing the viral RNA-mediated apoptosis of infected cells and the arrest of viral production.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Interferon β (IFN-β) Production during the Double-stranded RNA (dsRNA) Response in Hepatocytes Involves Coordinated and Feedforward Signaling through Toll-like Receptor 3 (TLR3), RNA-dependent Protein Kinase (PKR), Inducible Nitric Oxide Synthase (iNOS), and Src Protein.J Biol Chem. 2016 Jul 15;291(29):15093-107. doi: 10.1074/jbc.M116.717942. Epub 2016 May 17. J Biol Chem. 2016. PMID: 27226571 Free PMC article.
-
Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells.Cell Death Dis. 2013 Jan 31;4(1):e479. doi: 10.1038/cddis.2013.5. Cell Death Dis. 2013. PMID: 23370279 Free PMC article.
-
RNase L Amplifies Interferon Signaling by Inducing Protein Kinase R-Mediated Antiviral Stress Granules.J Virol. 2020 Jun 16;94(13):e00205-20. doi: 10.1128/JVI.00205-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295917 Free PMC article.
-
Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing.Immunol Rev. 2009 Jan;227(1):44-53. doi: 10.1111/j.1600-065X.2008.00723.x. Immunol Rev. 2009. PMID: 19120474 Review.
-
Antiviral responses induced by the TLR3 pathway.Rev Med Virol. 2011 Mar;21(2):67-77. doi: 10.1002/rmv.680. Epub 2011 Feb 10. Rev Med Virol. 2011. PMID: 21312311 Review.
Cited by
-
Susceptibility and Permissivity of Zebrafish (Danio rerio) Larvae to Cypriniviruses.Viruses. 2023 Mar 17;15(3):768. doi: 10.3390/v15030768. Viruses. 2023. PMID: 36992477 Free PMC article.
-
The Effect of Bovine Viral Diarrhoea Virus Biotypes on Bovine Oocyte In Vitro.Vet Med Sci. 2025 May;11(3):e70216. doi: 10.1002/vms3.70216. Vet Med Sci. 2025. PMID: 40205966 Free PMC article.
-
HSV-1 Triggers an Antiviral Transcriptional Response during Viral Replication That Is Completely Abrogated in PKR-/- Cells.Pathogens. 2023 Sep 3;12(9):1126. doi: 10.3390/pathogens12091126. Pathogens. 2023. PMID: 37764935 Free PMC article.
-
Oxymatrine Modulation of TLR3 Signaling: A Dual-Action Mechanism for H9N2 Avian Influenza Virus Defense and Immune Regulation.Molecules. 2024 Apr 24;29(9):1945. doi: 10.3390/molecules29091945. Molecules. 2024. PMID: 38731436 Free PMC article.
-
DNA-RNA hybrids in inflammation: sources, immune response, and therapeutic implications.J Mol Med (Berl). 2025 May;103(5):511-529. doi: 10.1007/s00109-025-02533-0. Epub 2025 Mar 25. J Mol Med (Berl). 2025. PMID: 40131443 Review.
References
-
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

