Design and synthesis of new N-thioacylated ciprofloxacin derivatives as urease inhibitors with potential antibacterial activity
- PMID: 35970866
- PMCID: PMC9378659
- DOI: 10.1038/s41598-022-17993-4
Design and synthesis of new N-thioacylated ciprofloxacin derivatives as urease inhibitors with potential antibacterial activity
Abstract
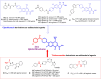
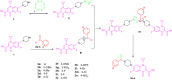
A new series of N-thioacylated ciprofloxacin 3a-n were designed and synthesized based on Willgerodt-Kindler reaction. The results of in vitro urease inhibitory assay indicated that almost all the synthesized compounds 3a-n (IC50 = 2.05 ± 0.03-32.49 ± 0.32 μM) were more potent than standard inhibitors, hydroxyurea (IC50 = 100 ± 2.5 μM) and thiourea (IC50 = 23 ± 0.84 μM). The study of antibacterial activity against Gram-positive species (S. aureus and S. epidermidis) revealed that the majority of compounds were more active than ciprofloxacin as the standard drug, and 3h derivative bearing 3-fluoro group had the same effect as ciprofloxacin against Gram-negative bacteria (P. aeruginosa and E. coli). Based on molecular dynamic simulations, compound 3n exhibited pronounced interactions with the critical residues of the urease active site and mobile flap pocket so that the quinolone ring coordinated toward the metal bi-nickel center and the essential residues at the flap site like His593, His594, and Arg609. These interactions caused blocking the active site and stabilized the movement of the mobile flap at the entrance of the active site channel, which significantly reduced the catalytic activity of urease. Noteworthy, 3n also exhibited IC50 values of 5.59 ± 2.38 and 5.72 ± 1.312 µg/ml to inhibit urease enzyme against C. neoformans and P. vulgaris in the ureolytic assay.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
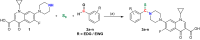
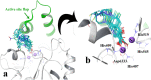

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

