Whole exome and genome sequencing in mendelian disorders: a diagnostic and health economic analysis
- PMID: 35970915
- PMCID: PMC9553973
- DOI: 10.1038/s41431-022-01162-2
Whole exome and genome sequencing in mendelian disorders: a diagnostic and health economic analysis
Abstract
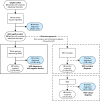
Whole genome sequencing (WGS) improves Mendelian disorder diagnosis over whole exome sequencing (WES); however, additional diagnostic yields and costs remain undefined. We investigated differences between diagnostic and cost outcomes of WGS and WES in a cohort with suspected Mendelian disorders. WGS was performed in 38 WES-negative families derived from a 64 family Mendelian cohort that previously underwent WES. For new WGS diagnoses, contemporary WES reanalysis determined whether variants were diagnosable by original WES or unique to WGS. Diagnostic rates were estimated for WES and WGS to simulate outcomes if both had been applied to the 64 families. Diagnostic costs were calculated for various genomic testing scenarios. WGS diagnosed 34% (13/38) of WES-negative families. However, contemporary WES reanalysis on average 2 years later would have diagnosed 18% (7/38 families) resulting in a WGS-specific diagnostic yield of 19% (6/31 remaining families). In WES-negative families, the incremental cost per additional diagnosis using WGS following WES reanalysis was AU$36,710 (£19,407;US$23,727) and WGS alone was AU$41,916 (£22,159;US$27,093) compared to WES-reanalysis. When we simulated the use of WGS alone as an initial genomic test, the incremental cost for each additional diagnosis was AU$29,708 (£15,705;US$19,201) whereas contemporary WES followed by WGS was AU$36,710 (£19,407;US$23,727) compared to contemporary WES. Our findings confirm that WGS is the optimal genomic test choice for maximal diagnosis in Mendelian disorders. However, accepting a small reduction in diagnostic yield, WES with subsequent reanalysis confers the lowest costs. Whether WES or WGS is utilised will depend on clinical scenario and local resourcing and availability.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

