Long non-coding RNA DARS-AS1 promotes tumor progression by directly suppressing PACT-mediated cellular stress
- PMID: 35970927
- PMCID: PMC9378715
- DOI: 10.1038/s42003-022-03778-y
Long non-coding RNA DARS-AS1 promotes tumor progression by directly suppressing PACT-mediated cellular stress
Abstract
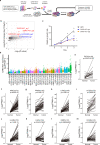
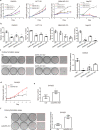
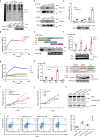
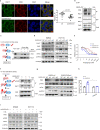
Cancer cells evolve various mechanisms to overcome cellular stresses and maintain progression. Protein kinase R (PKR) and its protein activator (PACT) are the initial responders in monitoring diverse stress signals and lead to inhibition of cell proliferation and cell apoptosis in consequence. However, the regulation of PACT-PKR pathway in cancer cells remains largely unknown. Herein, we identify that the long non-coding RNA (lncRNA) aspartyl-tRNA synthetase antisense RNA 1 (DARS-AS1) is directly involved in the inhibition of the PACT-PKR pathway and promotes the proliferation of cancer cells. Using large-scale CRISPRi functional screening of 971 cancer-associated lncRNAs, we find that DARS-AS1 is associated with significantly enhanced proliferation of cancer cells. Accordingly, knocking down DARS-AS1 inhibits cell proliferation of multiple cancer cell lines and promotes cancer cell apoptosis in vitro and significantly reduces tumor growth in vivo. Mechanistically, DARS-AS1 directly binds to the activator domain of PACT and prevents PACT-PKR interaction, thereby decreasing PKR activation, eIF2α phosphorylation and inhibiting apoptotic cell death. Clinically, DARS-AS1 is broadly expressed across multiple cancers and the increased expression of this lncRNA indicates poor prognosis. This study elucidates the lncRNA DARS-AS1 directed cancer-specific modulation of the PACT-PKR pathway and provides another target for cancer prognosis and therapeutic treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
DARS-AS1: A Vital Oncogenic LncRNA Regulator with Potential for Cancer Prognosis and Therapy.Int J Med Sci. 2024 Jan 20;21(3):571-582. doi: 10.7150/ijms.90611. eCollection 2024. Int J Med Sci. 2024. PMID: 38322590 Free PMC article. Review.
-
Oncogenic Long Noncoding RNA DARS-AS1 in Childhood Acute Myeloid Leukemia by Binding to microRNA-425.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820965580. doi: 10.1177/1533033820965580. Technol Cancer Res Treat. 2020. PMID: 33073700 Free PMC article.
-
DARS-AS1 promotes clear cell renal cell carcinoma by sequestering miR-194-5p to up-regulate DARS.Biomed Pharmacother. 2020 Aug;128:110323. doi: 10.1016/j.biopha.2020.110323. Epub 2020 Jun 8. Biomed Pharmacother. 2020. PMID: 32526457
-
Contribution of the two dsRBM motifs to the double-stranded RNA binding and protein interactions of PACT.J Cell Biochem. 2018 Apr;119(4):3598-3607. doi: 10.1002/jcb.26561. Epub 2018 Jan 4. J Cell Biochem. 2018. PMID: 29231267
-
The role of long non-coding RNA AFAP1-AS1 in human malignant tumors.Pathol Res Pract. 2018 Oct;214(10):1524-1531. doi: 10.1016/j.prp.2018.08.014. Epub 2018 Aug 20. Pathol Res Pract. 2018. PMID: 30173945 Review.
Cited by
-
DARS-AS1: A Vital Oncogenic LncRNA Regulator with Potential for Cancer Prognosis and Therapy.Int J Med Sci. 2024 Jan 20;21(3):571-582. doi: 10.7150/ijms.90611. eCollection 2024. Int J Med Sci. 2024. PMID: 38322590 Free PMC article. Review.
-
Proteomic and metabolomic signatures of U87 glioblastoma cells treated with cisplatin and/or paclitaxel.Ann Med. 2023;55(2):2305308. doi: 10.1080/07853890.2024.2305308. Epub 2024 Jan 22. Ann Med. 2023. PMID: 38253025 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

