The Tommy's Clinical Decision Tool, a device for reducing the clinical impact of placental dysfunction and preterm birth: protocol for a mixed-methods early implementation evaluation study
- PMID: 35971107
- PMCID: PMC9377101
- DOI: 10.1186/s12884-022-04867-w
The Tommy's Clinical Decision Tool, a device for reducing the clinical impact of placental dysfunction and preterm birth: protocol for a mixed-methods early implementation evaluation study
Abstract
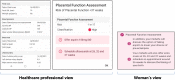
Background: Disparities in stillbirth and preterm birth persist even after correction for ethnicity and social deprivation, demonstrating that there is wide geographical variation in the quality of care. To address this inequity, Tommy's National Centre for Maternity Improvement developed the Tommy's Clinical Decision Tool, which aims to support the provision of "the right care at the right time", personalising risk assessment and care according to best evidence. This web-based clinical decision tool assesses the risk of preterm birth and placental dysfunction more accurately than current methods, and recommends best evidenced-based care pathways in a format accessible to both women and healthcare professionals. It also provides links to reliable sources of pregnancy information for women. The aim of this study is to evaluate implementation of Tommy's Clinical Decision Tool in four early-adopter UK maternity services, to inform wider scale-up.
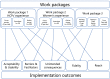
Methods: The Tommy's Clinical Decision Tool has been developed involving maternity service users and healthcare professionals in partnership. This mixed-methods study will evaluate: maternity service user and provider acceptability and experience; barriers and facilitators to implementation; reach (whether particular groups are excluded and why), fidelity (degree to which the intervention is delivered as intended), and unintended consequences. Data will be gathered over 25 months through interviews, focus groups, questionnaires and through the Tommy's Clinical Decision Tool itself. The NASSS framework (Non-adoption or Abandonment of technology by individuals and difficulties achieving Scale-up, Spread and Sustainability) will inform data analysis.
Discussion: This paper describes the intervention, Tommy's Clinical Decision Tool, according to TiDIER guidelines, and the protocol for the early adopter implementation evaluation study. Findings will inform future scale up.
Trial registration: This study was prospectively registered on the ISRCTN registry no. 13498237 , on 31st January 2022.
Keywords: Decision support; Implementation; Pregnancy; Preterm; Process evaluation; Risk assessment; Stillbirth; eHealth.
© 2022. The Author(s).
Conflict of interest statement
JS is Head of Maternity and Midwifery Research at NHS England and NHS Improvement. EL is a member of the University of Bristol (UoB) and part of his salary until 31/7/2021 was paid by the PROMPT Maternity Foundation (PMF) to UoB. He has no conflict of interest since then. All other authors declare that they have no competing interests.
Figures


Similar articles
-
A continuity of care programme for women at risk of preterm birth in the UK: Process evaluation of a hybrid randomised controlled pilot trial.PLoS One. 2023 Jan 12;18(1):e0279695. doi: 10.1371/journal.pone.0279695. eCollection 2023. PLoS One. 2023. PMID: 36634125 Free PMC article. Clinical Trial.
-
Implementation and evaluation of a quality improvement initiative to reduce late gestation stillbirths in Australia: Safer Baby Bundle study protocol.BMC Pregnancy Childbirth. 2020 Nov 13;20(1):694. doi: 10.1186/s12884-020-03401-0. BMC Pregnancy Childbirth. 2020. PMID: 33187483 Free PMC article.
-
Saving babies' lives project impact and results evaluation (SPiRE): a mixed methodology study.BMC Pregnancy Childbirth. 2018 Jan 30;18(1):43. doi: 10.1186/s12884-018-1672-x. BMC Pregnancy Childbirth. 2018. PMID: 29378526 Free PMC article.
-
Cue-based versus scheduled feeding for preterm infants transitioning from tube to oral feeding: the Cubs mixed-methods feasibility study.Health Technol Assess. 2021 Dec;25(74):1-146. doi: 10.3310/hta25740. Health Technol Assess. 2021. PMID: 34878383
-
What factors hinder the decision-making process for women with cancer and contemplating fertility preservation treatment?Hum Reprod Update. 2017 Jul 1;23(4):433-457. doi: 10.1093/humupd/dmx009. Hum Reprod Update. 2017. PMID: 28510760 Review.
Cited by
-
Formative research to optimize pre-eclampsia risk-screening and prevention (PEARLS): study protocol.Reprod Health. 2025 Mar 24;22(1):44. doi: 10.1186/s12978-025-01980-9. Reprod Health. 2025. PMID: 40128812 Free PMC article.
-
Disparities in Stillbirths in England: Analysis of A Population-Based Study of 1.3 Million Births.BJOG. 2025 Jul;132(8):1130-1138. doi: 10.1111/1471-0528.18147. Epub 2025 May 16. BJOG. 2025. PMID: 40376868 Free PMC article.
-
INSIGHT-2: mechanistic studies into pregnancy complications and their impact on maternal and child health-study protocol.Reprod Health. 2024 Nov 28;21(1):177. doi: 10.1186/s12978-024-01911-0. Reprod Health. 2024. PMID: 39609862 Free PMC article.
References
-
- Aughey H, Blotkamp A, Carroll F, Geary R, Gurol-Urganci I, Harris T, Hawdon J, Heighway E, Jardine J, Knight H, Mamza L. National Maternity and Perinatal Audit: Clinical report 2019. Based on births in NHS maternity services between 1 April 2016 and 31 March 2017.London: RCOG; 2019 Oct. Available from: https://maternityaudit.org.uk/pages/reports.
-
- Alderwick H, Dixon J. NHS The Long Term Plan. British Medical Journal Publishing Group; 2019. Available from: www.longtermplan.nhs.uk/publication/nhs-long-term-plan/.
-
- NHS England. Saving Babies Lives: a care bundle for reducing stillbirths. 2016. Available from: https://www.england.nhs.uk/wp-content/uploads/2016/03/saving-babies-live....
-
- NHS England. Saving Babies’ Lives Version 2: a care bundle for reducing perinatal mortality. 2019. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/07/saving-babies-live....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

